 download article
download articlepH Measurement in industry
Article index
 | Definition of pH | |
 | pH measuring system | |
 | pH combination electrode | |
 | Reference electrode | |
 | The effect of temperature | |
 | Calibration |
range works than to keep the pH within a desired range. It is therefore important that the person planning, making or using pH measurements understands how correct measurement is made, how tocalibrate the transmitter, and how to recognize and avoid common problems or limits.
Definition of pH
A pH value is defined as the negative logarithm of the hydrogen ion activity aH+pH = -log aH+
or in simple terms: pH is a number that describes the degree of acidity (H+) or alkalinity (OH-) of a solution.
The pH scale is determined by the dissocation of water.
H2O ↔ H+ OH-
The activities of these two ions are interrelated by the ionic product (l)
I = (aH+)*(aOH-) = 10-14
(at 25 ºC / 77 ºF )
(See Fig. 1: pH concentration relationship)
| RANGE | pH | H+ concentration (mol / l) | OH- concentration (mol / l) |
| 0 | 1 | 0.000 000 000 000 01 | |
| 1 | 0.1 | 0.000 000 000 000 1 | |
| 2 | 0.01 | 0.000 000 000 001 | |
| Acid | 3 | 0.001 | 0.000 000 000 01 |
| 4 | 0.000 1 | 0.000 000 000 1 | |
| 5 | 0.000 01 | 0.000 000 001 | |
| 6 | 0.000 001 | 0.000 000 01 | |
| Neutral | 7 | 0.000 000 1 | 0.000 000 1 |
| 8 | 0.000 000 01 | 0.000 001 | |
| 9 | 0.000 000 001 | 0.000 01 | |
| 10 | 0.000 000 000 1 | 0.000 1 | |
| 11 | 0.000 000 000 01 | 0.001 | |
| 12 | 0.000 000 000 001 | 0.01 | |
| 13 | 0.000 000 000 000 1 | 0.1 | |
| Alkaline | 14 | 0.000 000 000 000 01 | 1 |
pH measuring system
The conventional sensor for pH measurement is a pH glass electrode, combined with a reference electrode and a temperature sensor. An electrode housing is necessary in order to introduce the pH electrode into the medium of a (continuous) industrial process and to protect it during operation.The function of the transmitter is to interpret the signals from the electrode in a suitable way, perform proper temperature compensation, display the pH value, and transmit an output signal to next level system components.
pH combination electrode
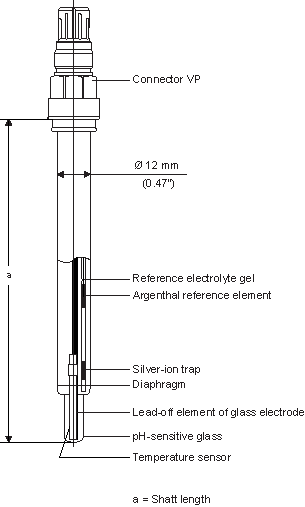
(Fig. 2: Typical construction)
Fig. 2 shows a typical construction of a pH combination electrode, when an electrode comes into contact with an aqueous measuring solution, a gel layer develops on the pH sensitive glass membrane. Such a gel layer also forms on the inside of the glass membrane wich is in contact with a defined buffer solution (inner buffer). The H+ ions diffuse out or into the gel layer depending on the pH value of the measured solution. The total membrane potential is a result of the difference between inner and outer charge.
The whole pH measuring circuit (Fig. 3) consists of a measuring electrode and a reference electrode wich has a defined potential independent of the sample solution.
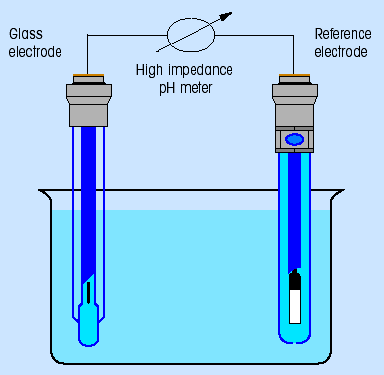
(Fig. 3: pH measuring circuit)
The resistance of the pH sensitive glass increases greatly at decreased temperatures (Fig. 4).
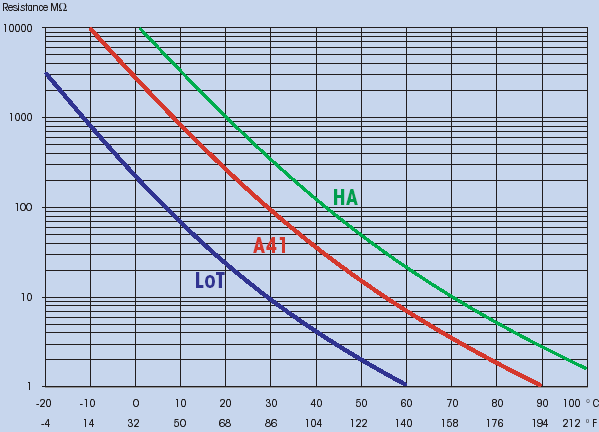
(Fig. 4: Resistance of pH-sensitive glass in relation to temperature)
Reference electrode
The reference electrode consists of a reference element (Ag / AgCl) with silver-ion barier wich is immersed in a defined electrolyte containing chloride ions. This electrolyte must be in contact with the measured solution trough a liquid junction.Porous ceramic junctions are most common. Xerolyt polymer electrode has an open aperture as a liquid junction and this direct contact of measuring solution with the polymer electrolyte provides superior protection against junction clogging.
The best advice for reliable pH measurement is to keep the liquid junction clean.
The effect of temperature
Temperature variation can influence measurement accuracy:1. At temperature variations of the liquid being measured
2. Trough effects of temperature variations on the contact potential in the glass and the reference system.
To 1: effects of temperature on the process liquid
Chemical equilibria are temperature-dependent and therefore the pH value of a given measuring solution is itself temperature-dependent. For accurate comparison of pH values made by different techniques (e.g. by lab measurement of a grab sample or industrial (in-line) measurement), it is necessary to carry out the two measurements at indentical temperatures.
To 2: effects of temperature on the electrode
This temperature-dependency is described by the Nernst equation, graphically shown in Fig. 5.
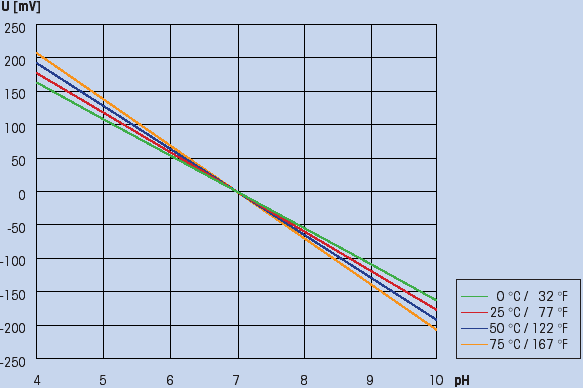
(Fig. 5: Temperature effect on the electrode)
This graph shows that:
-The mV / pH ratio increases as the temperature increases. At 25 ºC (77 ºF) the theoretical slope (100%) is 59.16 mV / pH and at 80 ºC (176 ºF) 70.80 mV / pH
-The various isothermal lines intersect at one point (the isothermal point of intersection).
The temperature-dependency of a pH combination electrode can be easily compensated for by the automatic temperature compensation function of the transmitter. To achieve this, the electrode is equipped with an integrated temperature-sensing element (Pt 100 or Pt 1000), or a separate temperature sensor is connected to the transmitter. Without temperature compensation errors like those shown in Fig. 6 are to be expected.
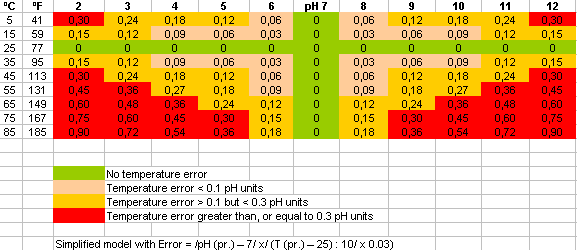
(Fig. 6: Temperature error table for pH signal)
Calibration
Both the zero point, i.e. the point where the pH electrode delivers 0mV, and the slope of the calibration line show circumstancedependent tolerances and will change after exposure to the measuring solution. Therefore the pH electrode (respectively the pH transmitter) has to be calibrated with accurately defined buffer solutions.The zero point can be set by using buffer pH 7, and the slope can be calibrated with a second buffer (a difference in pH values of at least two pH units). The stability of the zero and the slope depends on the composition of the measuring solution as well as on the temperature. When working with unknown solutions, it is advisable to frequently repeat calibration at the beginning. The use of a retractable housing allows calibration or cleaning of the electrode without interrupting the on-going process.
 back to top
back to top


 companies
companies
