Dit artikel is nog niet beschikbaar in de door u gekozen taal
2 Risk management
Inhoudsopgave
Summary
This chapter provides a comprehensive overview of the management of food-borne risks to consumers. A generic risk management framework (RMF) is described in some detail. The RMF consists of four steps: i) preliminary risk management activities; ii) identification and selection of risk management options; iii) implementation; and iv) monitoring and review. Where necessary and feasible, a risk assessment is commissioned within the RMF as a functionally separate exercise (Chapter 3 Risk assessment ). Most stages of risk management require extensive communication, coordination and collaboration, both between risk managers and risk assessors, and with external stakeholders (Chapter 4 Risk communication ). Application of each step in the RMF is illustrated by examples of management for chemical and microbiological food-borne risks at the national and international levels.2.1. Introduction
Risk analysis must occur in a context and, to be done effectively, requires a formal process. In a typical instance, a food safety problem or issue is identified and risk managers5 initiate a risk management process, which they then see through to completion. This is best accomplished within a systematic, consistent and readily-understood framework in which scientific knowledge on risk and evaluations of other factors relevant to public health protection are used to select and implement appropriate control measures. The responsibilities of risk managers during this process also include commissioning a risk assessment when one is needed, and making sure that risk communication occurs wherever necessary.The generic risk management framework (RMF) presented in this Guide provides a practical, structured process for food safety regulators to apply all the components of risk analysis. It is comprised of four major phases and numerous specific activities (see Figure 2.1). The complete process is cyclical and there may be many iterative loops between phases and steps. Parts of the RMF can be repeated as new information becomes available, or as work done at a later phase indicates a need to modify or re-examine work done at an earlier stage.
2.1.1. Perspectives on risk
Food safety risks can be viewed in several ways (Box 2.1) and each of these perspectives may be applied by some participants in any given application of the food safety RMF. The “technical” view is the primary one for decision-making, but risk managers also apply psychological and sociological risk perspectives, as appropriate, in establishing food safety standards. As described in the next chapter Risk assessment, food safety risk assessment is anchored to the greatest extent possible in the technical perspective, and risk assessors are expected to base their work on scientific data and methods. The overriding consideration in the technical paradigm is that risk assessment is specific to the described scenario.| Box 2.1. Perspectives on risk |
|
| Technical paradigm: | Focuses on and is limited to scientific evaluation of the likelihood and severity of harm. May include an economic subset in which harm can be described in terms of either health indices, such as Disability Adjusted Life Years (DALYs) or monetary values. |
| Psychological paradigm: | Evaluates risk as a function of individual perception, giving weight to such attributes as voluntariness of exposure, controllability of risk, catastrophic nature of risk, and so on. Risk perceived in these ways may differ in “magnitude” from technical risk estimates. |
| Sociological paradigm: | Views risk as a social and cultural construct, with the goal of distributing costs and benefits in socially acceptable and equitable ways. |
2.2. A generic risk management framework
A generic process for carrying out risk management is presented in Figure 2.1. Such frameworks developed at the international level (e.g. the Codex Committee on Food Hygiene (CCFH) has developed principles and guidelines for the conduct of microbiological risk management6) provide useful templates for countries developing their own risk management systems.A generic RMF for food safety risk management must be functional in both strategic, long-term situations (e.g. development of international and national standards when sufficient time is available) and in the shorter term work of national food safety authorities (e.g. responding rapidly to a disease outbreak). In all cases, it is necessary to strive to obtain the best scientific information available. In the former situation, risk managers will usually have access to extensive scientific information in the form of risk assessment reports. In the latter situation, risk managers are not likely to have access to a complete risk assessment and therefore will need to rely on whatever scientific information on risks is readily available (such as human health surveillance and food-borne disease outbreak data) as a basis for preliminary decisions on control measures.
Figure 2.1. Generic framework for risk management
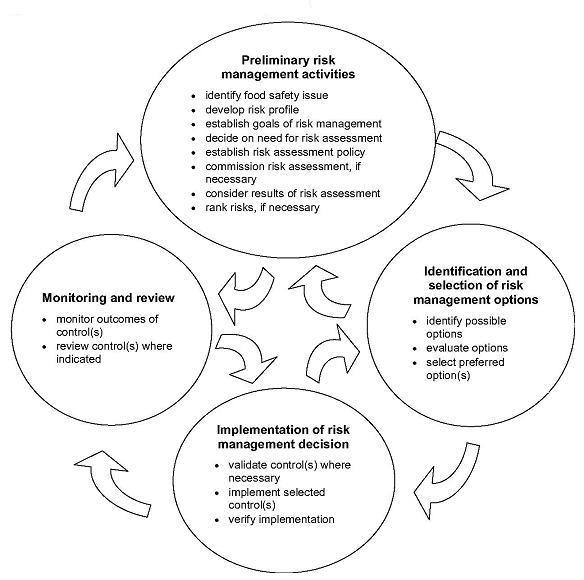
2.3. Understanding risk management
The first phase of the RMF shown in Figure 2.1 consists of “preliminary risk management activities”. After a food safety issue has been identified, available scientific information is aggregated into a risk profile that will guide further action. Risk managers may seek additional and more detailed scientific information on an assessment of risks from methodologies such as risk assessment, risk ranking or epidemiology-based approaches such as source attribution. Ranking using tools (see section 3.2.2) that rely on knowledge of risk factors to rank risks and prioritize regulatory controls may be carried out either within or without risk assessments. Epidemiology (see section 3.2.3) includes observational studies of human illness such as case-control, analysis of surveillance data and focused research, and is used to apportion risks and contribute to setting risk-based standards. These approaches are often used in combination.If a risk assessment is needed, it can be commissioned from those responsible for that function, with iterative discussions between risk managers and risk assessors to determine the scope of the risk assessment and to decide on questions it is to answer. Near the end of this preliminary stage, the results of the risk assessment are delivered back to the risk managers and further discussions are generally held on the results and their interpretation.
During this “preliminary” phase, good risk communication is important. Communication with external interested parties often is needed to fully identify the food safety issue, obtain sufficient scientific information for risk profiling, and formulate questions to be answered by the risk assessment. Internal communication between risk managers and risk assessors is vital for many reasons, such as to ensure that the scope of the risk assessment is reasonable and achievable, and that the results are presented in a readily understandable form.
The second phase of the RMF consists of identifying and evaluating a variety of possible options for managing (e.g. controlling, preventing, reducing, eliminating or in some other manner mitigating) the risk. As before, effective communication is a prerequisite for success, as information from and opinions of affected stakeholders, particularly industry and consumers, are valuable inputs to the decision-making process.
Weighing the results of the risk assessment as well as any economic, legal, ethical, environmental, social and political factors associated with the risk-mitigating measures that might be implemented can be a complex task. Economic evaluation of possible risk management interventions enables risk managers to examine the health impacts and feasibility of a proposed intervention relative to its cost. An open and participatory process helps ensure that the final decision is understood and widely supported by those affected by it.
When preferred risk management options have been selected, they must be implemented by the relevant stakeholders. In many countries today, industry has the primary responsibility for implementing regulatory standards. However, some non-regulatory risk management options may be selected, such as quality assurance schemes at the farm level, or consumer education packages for food handling in the home. Generally, national food safety authorities must validate and verify implementation of regulatory standards.
Once control measures have been implemented, monitoring and review activities should be carried out. The goal is to determine whether the measures that were selected and implemented are in fact achieving the risk management goals they were meant to achieve, and whether they are having any other unintended effects. Both industry and government bodies are likely to be involved in monitoring and review activities. Both sectors usually monitor levels of hazard control, while government generally carries out health surveillance of the population to determine the level of food-borne illness. If monitoring information indicates a need to review the decision as to risk management options, the risk management process can begin a new cycle, with all interested parties participating as appropriate.
When dealing with a given specific food safety issue, a RMF can be entered at any phase and the cyclical process can be repeated as many times as is necessary. What is most important is that appropriate attention is paid to all the phases in the process. More than anything else, application of the RMF represents a systematic way of thinking about all food safety issues that require risk management. The level of intensity of each phase will be matched to the needs presented by each food safety issue and may range from simple, qualitative processes to complex scientific and social evaluations.
The succeeding sections of this chapter examine step-by-step application of the risk management framework, as described above.
2.4. Preliminary risk management activities7
2.4.1. Step 1: Identify and describe the food safety issue
Identifying and articulating the nature and characteristics of the food safety issue is an essential first task for risk managers. Sometimes the issue is already recognized and accepted as a food safety problem that needs formal risk assessment. At other times, the problem may be apparent but additional information is needed before further actions can be decided on and implemented.A RMF can also be used to resolve food safety issues that do not necessarily require risk reduction (see Box 2.2). For example, as new processing technologies such as gas depelting of fresh meat carcasses become available, it is necessary to see whether these innovations produce any changes in bacterial contamination profiles that might affect the current level of consumer protection. In other situations, new technologies may require interventions to avoid increased risks. For instance, in the early stages of the BSE epidemic in the United Kingdom, the use of mechanical separation of muscle from bone in meat packing houses needed to be re-evaluated because this method commingles nervous tissue (a specific risk material) with meat fragments.
Food safety authorities learn about food safety issues that require resolution in a variety of ways. Safety problems may be identified by domestic and international (point of entry) inspection, food monitoring programmes, environmental monitoring, laboratory,
Box 2.2. Some food safety issues that benefit from application of a RMF
|
A brief initial description of the food safety issue provides the basis for developing a risk profile, which in turn generates a context and guide for further action. This first step also usually requires risk managers to determine their initial public health objectives. If the problem is urgent and solutions must be implemented rapidly, any risk analysis may be limited and the range of options considered may be fairly restricted. For less urgent problems, the scope of a risk analysis could potentially be very wide. But resource limitations, legal and political considerations, and other factors generally help risk managers make practical decisions about the depth and length of the risk analysis that is to be conducted in any given case.
Box 2.3. Examples of Step 1: Identifying a food safety issue
|
2.4.2. Step 2: Develop a risk profile
A risk profile requires gathering relevant information on an issue and may take a number of forms. Its main purpose is to assist risk managers in taking further action. The extent of the information gathered can vary from case to case but should always be sufficient to guide the risk managers in determining the need for (and if needed, the extent of) a risk assessment. Risk managers are generally unlikely to carry out risk profiling themselves unless the food safety issue is urgent and there is a need for immediate action. Ordinarily, a risk profile is developed primarily by risk assessors and others with specific technical expertise on the issue(s) at hand.A typical risk profile includes a brief description of: the situation, product or commodity involved; information on pathways by which consumers are exposed to the hazard; possible risks associated with that exposure; consumer perceptions of the risks; and the distribution of possible risks among different segments of the population. By gathering available information on risks, the risk profile should assist risk managers in setting work priorities, deciding how much further scientific information on the risks is needed, and developing a risk assessment policy. By describing current control measures, including those in place in other countries where relevant, the risk profile can also assist risk managers in identifying possible risk management options. In many situations, a risk profile can be thought of as a preliminary risk assessment that summarizes everything the risk managers know about the possible risks at that time. Examples of risk profiles are given Box 2.4.
| Box 2.4. Examples of Step 2: Developing a risk profile The New Zealand Food Safety Authority (NZFSA) has developed risk profiles for a large number of food-borne hazards, and they are posted on the authority’s web site (http://www.nzfsa.govt.nz/science/risk-profiles/index.htm). Profiles for new hazard-food combinations are added to the library year-by-year. Profiles now posted address primarily microbiological contaminants of foods, including Salmonella and Campylobacter in poultry, Listeria in ice cream and ready-to-eat meats, and an array of other hazards. On the chemical side, NZFSA has developed risk profiles on aflatoxins in maize and glyphosate (an herbicide residue) in soy and soy products. For detailed illustrations of the kinds and amounts of information contained in a risk profile, readers are invited to examine the NZFSA examples. The case studies on methylmercury in fish and Listeria monocytogenes in ready-to-eat foods, in Annexes 2 and 3 of this Guide, include brief descriptions of risk profiles. |
A good risk profile provides the basis for commissioning a risk assessment where this is deemed necessary and assists in identifying the questions that need to be answered by the risk assessment. Formulating these questions usually requires significant interaction between risk assessors and risk managers, as well as dialogue with appropriate external parties (e.g. those with relevant information about the potential hazard).
Some types of information that may be included in a risk profile are listed in Box 2.5. The risk profile should be clearly and thoroughly documented, so that risk managers can use it to decide on further action in relation to a specific food safety issue. If links are made between risk profiles for other hazard-food combinations, risk profiles can provide the basis for qualitative ranking of food safety problems for subsequent risk management.
2.4.3. Step 3: Establish broad risk management goals
Following development of the risk profile, risk managers need to decide on the broader risk management goals. This is likely to occur in conjunction with a decision on whether or not a risk assessment is feasible or necessary. Delineating risk management goals must precede commissioning of a risk assessment and determines at least some of the questions to be asked of, and possibly answered by, the risk assessment. Some generic risk management goals that may require a risk assessment to resolve a food safety issue are shown in Box 2.6.2.4.4. Step 4: Decide whether a risk assessment is necessary
Deciding whether a risk assessment is necessary is an iterative decision for risk managers and risk assessors and may be part of establishing broader risk management goals. Questions such as how a risk assessment might be approached, what questions it might try to answer, what methods might yield useful answers, and where data gaps or uncertainties might likely preclude clear-cut answers, are significant issues. If the risk managers decide to progress to commissioning a risk assessment to support their risk management objectives, addressing such matters is essential. Identifying key data gaps at the outset also facilitates essential information being gathered to the extent possible before and during the risk assessment. These activities usually require the cooperation of scientific institutions, research-oriented bodies and the industry concerned.A risk assessment is likely to be especially desirable when the nature and magnitude of the risk are not well characterized, when a risk brings multiple societal values into conflict or is a pressing public concern, or when risk management has major trade implications. A risk assessment also can guide research by facilitating the ranking of risks of most importance.
Box 2.5. Examples of information that may be included in a risk profile
|
Practical issues that impact on the decision as to whether a risk assessment is needed are: time and resources available; how urgently a risk management response is needed; consistency with responses to other similar issues; and availability of scientific information. If the risk profile indicates that food-borne risks are significant and immediate, the regulator may decide to impose interim regulatory control measures while a risk assessment is undertaken. On the other hand, some issues can be resolved simply and rapidly without need for a risk assessment. In some situations, a specific regulatory response will be deemed unnecessary because of the limited nature of possible risks. Box 2.7 offers some examples of cases in which a risk assessment is or is not likely to be needed.
Box 2.6. Examples of generic risk management goals that may require a risk assessment to resolve a food safety issue
|
2.4.5. Step 5: Establish a risk assessment policy
Many subjective judgements and choices arise in the course of a risk assessment, and some of those choices will affect the utility of the assessment’s results for decision making. Other choices may involve scientific values and preferences, such as how to deal with uncertainty and what assumptions to use when the available data are inconsistent, or how much caution to apply when recommending acceptable exposures.8 See Chapter 3 Risk assessment, section 3.3.4, for a more detailed discussion and examples of some of the “inferential bridges” that may be necessary for a risk assessment to proceed.Box 2.7. Examples of Step 4: Deciding whether a risk assessment is needed
|
A policy is often developed to provide an agreed framework for the conduct of risk assessment. Risk assessment policy is defined in the 15th Edition of the Codex Alimentarius Commission Procedural Manual as “documented guidelines on the choice of options and associated judgements for their application at appropriate decision points in the risk assessment such that the scientific integrity of the process is maintained”. While establishing risk assessment policy is a responsibility of risk managers, it should be carried out in full collaboration with risk assessors, through an open and transparent process that allows appropriate inputs from relevant stakeholders. Risk assessment policy should be documented to ensure consistency, clarity and transparency.
A risk assessment policy underpins a clear understanding of the scope of the risk assessment and the manner in which it will be conducted. It often defines the parts of the food system, the populations, geographic areas and the time period to be covered. A risk assessment policy may include criteria for ranking risks (where, for example, the assessment covers different risks posed by the same contaminant, or risks posed by the contaminant in different foods) and procedures for applying uncertainty factors. Establishing a risk assessment policy provides guidance as to the appropriate level of protection and the scope of the risk assessment. An illustration is given in Box 2.8, and more details about risk assessment policy and examples from the perspective of the risk assessor are presented in Chapter 3 Risk assessment (section 3.3.4).
| Box 2.8. Example of Step 5: Establishing a risk assessment policy In the United States in 1996, Congress, acting as risk managers, established a new policy directing risk assessments by the United States Environmental Protection Agency (EPA) for pesticide residues in the diet. Legislation now requires the EPA to ensure that pesticide residue limits protect the most sensitive populations (infants and children); to apply an additional uncertainty factor when the evidence is insufficient to be reasonably certain that the standard uncertainty factors would ensure safety; and to consider the cumulative effects of multiple residues that share a common mechanism of toxic action, as well as exposures from water and home pesticide use, when defining tolerable exposure from food. |
2.4.6. Step 6: Commissioning the risk assessment
Once a decision is made that a risk assessment is required, risk managers must arrange to get the risk assessment done. The nature of the risk assessment and the method by which it is commissioned may vary, depending on the nature of the risk, the institutional context and resources available and other factors. In general, risk managers must assemble an appropriate team of experts to carry out the task, and then interact with the risk assessors extensively enough to instruct them clearly on the work to be performed, while maintaining a “functional separation” between risk assessment and risk management activities.Functional separation means separating out the tasks that are carried out as part of risk assessment or risk management at the time when they are being performed. While developed countries may have separate bodies and personnel to carry out risk assessment and risk management, in developing countries the same individuals may be responsible for both. What is important is that conditions are in place to ensure that the tasks are carried out separately of each other (even if they are performed by the same individuals) using existing structures and resources. Functional separation need not require the establishment of different bodies and personnel for risk management and risk assessment.
Box 2.9. Responsibilities of risk managers in commissioning and supporting a risk assessment
|
When ample time and resources are available, assembling an independent multidisciplinary team of scientists to conduct a risk assessment is often appropriate. In other cases, regulators may call on in-house expert resources or those available from dedicated external science providers, such as academic institutes. The most effective risk assessment teams are interdisciplinary; for instance, when dealing with a microbial hazard, the team may include food technologists, epidemiologists, microbiologists and biostatisticians.
Risk assessments carried out by the joint FAO/WHO expert bodies (JECFA, JMPR or JEMRA) are primarily intended to inform and assist the Codex Alimentarius Commission and governments in their choice of risk management measures for particular hazard-food combinations.9 Historically, many governments have directly used international risk assessment work by adopting Codex standards for chemical hazards in foods. In other cases, international risk assessments have been used as a starting point for further, nationally-specific risk assessments and establishing national standards for chemical hazards. In the case of microbial hazards, few international risk assessments are available but those that are provide an important aid in the establishment of standards at the national level.
National risk managers must ensure that a risk assessment is appropriately commissioned and carried out. Whatever the scope and nature of a risk assessment and regardless of the identity of the risk assessors and risk managers, certain principles should govern this critical step (see Box 2.9). Box 2.10 provides examples of how specific risk assessments were commissioned.
In practice, “functional separation” means that risk managers and risk assessors have different jobs to do, and they each need to do their own jobs. Risk managers must avoid the temptation to “guide” the risk assessment so that it supports a preferred risk management decision, and risk assessors must assemble and assess the evidence objectively, without being influenced by risk management concerns such as economic benefits of an activity, costs of reducing exposure or consumer perceptions of risks.
In some situations, where resources and legal frameworks permit or require it, risk assessments may be carried out by an independent scientific institution, distinct from a food control authority. In other cases, particularly in smaller countries or countries with limited resources, officials may of necessity serve in multiple roles with the same individuals carrying out both risk management and risk assessment tasks. Nevertheless, by striving to keep the two functions separate, and by following the principles outlined in Box 2.9, national risk managers can generally ensure that a risk assessment they commission is soundly conducted, objective and unbiased.
2.4.7. Step 7: Consider the results of the risk assessment
The risk assessment should clearly and fully answer the questions asked by the risk managers as far as possible given the availability of data and, where appropriate, identify and quantify sources of uncertainties in risk estimates. In judging the risk assessment complete, risk managers need to:- Be fully informed about the strengths and weaknesses of the risk assessment and its outputs.
- Be sufficiently familiar with the risk assessment techniques used, so that they can explain it adequately to external stakeholders.
- Understand the nature, sources and extent of uncertainties and variability in risk estimates.
- Be aware of and acknowledge all important assumptions made during the risk assessment and their impact on the results.
At this point in the preliminary risk management phase, when the risk assessment is complete and can be reviewed and discussed with interested parties, effective communication among risk managers, risk assessors and others with a stake in the issue is essential (see Chapter 4 Risk communication ).
2.4.8. Step 8: Rank food safety issues and set priorities for risk management10
National food safety authorities must deal with numerous food safety issues, often simultaneously. Resources inevitably are insufficient to manage all issues at any given time and ranking of issues in priority for risk management, as well as ranking risks for assessment, are important activities for food safety regulators.The primary criterion for ranking is generally the perceived relative level of risk each issue presents to consumers, so that risk management resources can be optimally applied to reduce overall food-borne public health risks. Issues may also be prioritized based on other factors, including serious restrictions in international trade resulting from different food safety control measures; the relative ease or difficulty of resolving the issues; and, sometimes, pressing public or political demand that attention be paid to a particular problem or issue. Application of risk ranking tools is described in more detail in Chapter 3 Risk assessment. The risk ranking exercise with Listeria in food in the United States (see Box 2.3) illustrates a case in which the relative risk per food category was totally different from the absolute risk.
| Box 2.10. Examples of Step 6: Commissioning a risk assessment Case study 1: Total aflatoxins in peanuts When aflatoxins were evaluated for the first time by the 31st session of JECFA in 1987, sufficient information was unavailable to establish a figure for a tolerable level of intake. At its 46th session, JECFA considered potency evaluations and population estimates and recommended that these analyses be completed and presented in an updated toxicological review. Concurrently, the Codex Committee on Food Additives and Contaminants had been considering the establishment of a maximum level for aflatoxins in peanuts for further processing for several sessions but could not reach consensus on a proposed maximum level of 15µg/kg. The 29th session of CCFAC (1997) asked JECFA, in the framework of its re-evaluation of aflatoxins, to consider the public health implications of a level of 15µg/kg, as compared to 10µg/kg, as these were the two levels under discussion. The 49th JECFA session (1997) completed the toxicological evaluation of aflatoxins and concluded that the potency of aflatoxins in individuals who carry the hepatitis B virus (HBsAg+) was substantially higher than in individuals who do not carry the virus. Reduction of the intake of aflatoxins in populations with a high prevalence of HBsAg+ individuals would therefore have greater impact on reducing liver cancer rates. The analysis of the application of hypothetical levels (10 µg/kg and 20 µg/kg aflatoxin in food) to model populations indicated that: i) populations with a low prevalence of HBsAg+ individuals and/or with a low mean intake are unlikely to exhibit demonstrable differences in population risks for levels in the range of the hypothetical cases; and ii) populations with a high prevalence of HBsAg+ individuals and high mean intake of aflatoxins would benefit from reductions in aflatoxin intake. As regards the two aflatoxin levels proposed, JECFA concluded that the higher level would yield almost identical liver cancer risks as the lower level. It indicated that “when a substantial fraction of the food supply is heavily contaminated, reducing the aflatoxin contamination levels may detectably lower cancer rates. Conversely, when only a small fraction of the food supply is heavily contaminated, reducing the level by an apparently substantial amount may have little appreciable effect o public health.” Taking into account the results of the JECFA evaluation, the CCFAC agreed on a maximum level of 15 µg/kg for total aflatoxins in peanuts for further processing, that was adopted, with the corresponding sampling plan, by the Codex Alimentarius Commission in 1999. Case study 2: Residues of nitrofurans* in prawns in Australia In 1993 JECFA withdrew the acceptable daily intake for four nitrofuran* chemicals (furazolidone, furaltadone, nitrofurantoine and nitrofurazone) due to the incomplete nature of the toxicological database and concerns about carcinogenicity in animal studies. As a result, several countries, including Australia, restricted, or prohibited, the use of nitrofurans in food-producing animals and subsequently, detectable residues in food products were not permitted. In October 2003, data became available indicating that very low levels of a furazolidone metabolite, 3-amino-oxazolidinone, had been found in certain imported prawns. Where residues had been detected, they were at a few parts per billion (µg/kg). However, in the absence of a specific maximum residue level in the Australian Food Standards Code, these residues were not permitted. As a result of these test findings, Food Standards Australia New Zealand (FSANZ) undertook a risk assessment to establish the level of food safety risk to consumers from the levels of residue being detected in prawns. The risk assessment was undertaken to help inform enforcement agencies as to whether any risk managements actions should be taken to protect consumer health, such as testing of prawns and/or recalls of batches of prawns containing detectable residues. The dietary exposure assessment component of the risk assessment utilized the residue concentrations found in an industry survey, and the hazard identification and characterization was based on a re-evaluation of the data summarized in the JECFA monographs. * Nitrofurans are synthetic broad-spectrum antimicrobial agents used in some countries in human and veterinary medicine. This example has been reproduced from a case study prepared by FSANZ (available at: http://www.fao.org/docrep/meeting/006/j1985e/j1985e00.htm). |
2.5. Selection of risk management options
The second major phase of the generic RMF (presented above in Figure 2.1) involves the identification, evaluation and selection of risk management options. Although this step ordinarily cannot be fully undertaken until a risk assessment has been completed, as a practical matter, it begins very early in a risk analysis, and is reiterated as information about the risk grows more complete and quantitative. A risk profile may contain some information about possible risk management measures (see Box 2.5 above), and when risk managers commission a risk assessment, they may ask specific questions, the answers to which may guide the choice among risk management options. Also, as discussed at Step 3 in section 2.4 above, in urgent food safety situations, it may be necessary to choose and implement at least some preliminary risk management measures before a risk assessment can be carried out.As was true for the first phase of risk management, this phase also consists of several distinct substeps. The exact order in which these activities are carried out is less important than the fact that they each take place.
2.5.1. Step 1: Identify available management options
Bearing in mind the risk management goals already established (see Step 3, section 2.4) and the outcome of the risk assessment, risk managers will generally identify a range of risk management options with the capacity to resolve the food safety issue at hand. The risk managers are responsible for the process that identifies appropriate measures, but need not always perform all the work themselves. Often risk assessors, scientists from food industry, economists and other stakeholders also play important roles in identifying options based on their expertise and knowledge. Examples of generic options for managing food-related risks (whether the hazards involved are chemical or microbiological) are illustrated in Box 2.11Box 2.11. Examples of generic approaches to identifying risk management options
|
The process of identifying options is conceptually simple but is often restricted by limits on food safety risk managers’ ability to implement selected options. While risk managers should try to take into account the entire continuum from production to consumption when identifying possible control measures (see Box 2.12), in many cases a particular regulatory agency has jurisdiction over only a segment of that continuum. In other situations, a risk assessment may be restricted to a small part of the food production chain and only measures within the scope of the risk assessment may be identified for possible implementation.
| Box 2.12. The production-to-consumption approach to risk management Food safety regulators in many countries are adopting a “production-to-consumption” approach to food safety. This approach strives to apply risk-based regulatory and non-regulatory control measures at appropriate points in the food production chain to achieve risk management goals in the most efficient and cost-effective manner. The approach assumes that basic good hygienic practices and good manufacturing practices are in place all along the food production chain and that opportunities exist to identify and implement targeted risk-reducing measures at relevant points along the continuum. Ideally, benefit-cost analysis and risk assessment are both conducted to inform risk management choices. The complexity of food production systems and the ever-changing nature of international trade in foods make it impractical to realize this approach fully in many situations. Certain inputs to food production, such as hazard profiles of animal feeds in different countries may change rapidly. Further, the administrative framework for national food control systems may not be integrated throughout the entire food production continuum. When risks are generated in one country, as during primary production of a food, but managed in another country, such as when specific characteristics of a high-susceptibility population subgroup in the importing country must be managed, basing risk-management decisions on benefit-cost analysis is often impractical. |
In some cases, a single measure may have the potential to successfully manage the risks associated with a particular food safety issue. In other cases, a combination of measures may be necessary. In some cases, a very limited range of risk management options may be available, over and above what is in place as good hygienic practice. In general, to the extent practicable, it is valuable to consider initially a relatively broad range of possible options, then to select the most promising alternatives for more detailed evaluation. It is also important at this stage to seek input from a variety of interested parties with knowledge of the food safety issue in question.
In some situations, effective control of a hazard in a particular part of a food production chain will require a systems approach, for example, control of faecal contamination of the carcass during the many steps in slaughter and dressing of red meat and poultry carcasses where this type of contamination can occur. Where a risk assessment process has identified the level of control required at the end of such a process, the risk management options may be integrated into a complete “food safety plan” based on a generic system such as HACCP, rather than described as distinct, narrower control measures.
2.5.2. Step 2: Evaluate the identified management options
The evaluation of identified risk management options is sometimes straightforward, for instance if the solution is obvious and relatively easy to implement, or if only a single option is under consideration. On the other hand, many food safety problems involve complex processes, and many potential risk management measures vary in feasibility, practicality and the degree of food safety they can achieve, and may require cost-benefit analysis and evaluating trade-offs among competing societal values.One of the most critical elements in evaluating and selecting food safety measures is to recognize that a clear link must be established between the risk management option being evaluated and the level of risk reduction and/or consumer protection that is provided (see Box 2.13).
| Box 2.13. “Risk-based” food safety measures Food safety measures based on risk assessments are generally designed to reduce risks to a target level, and risk managers must determine the degree of health protection they are aiming to achieve. Through good communication with risk managers, risk assessors will likely have examined the relative impacts of different controls on reducing risks, providing the risk managers with objective data that supports decisions on the most appropriate controls. The overriding objective of risk management is to maximize risk reduction while ensuring that the measures employed are efficient and effective and not overly restrictive. In this context, “risk-based” controls are formulated according to current knowledge about the human health risks associated with a food-borne hazard, whether expressed quantitatively or qualitatively. Control measures are aimed at achieving an established level of human health protection (which also may be expressed quantitatively or qualitatively) and should be explained and validated on those terms. For foods in international trade, the established level of consumer protection in the importing country is called the “appropriate level of protection” (ALOP). |
There are no strict rules about how to select the best options; rather, there are a variety of possibilities based on the food safety issue at hand and the risk management goals that apply. In the ideal situation, the following information should be available for evaluating individual or groups of possible risk management options:
- A “menu” of estimates of risk that would result from application of potential risk management measures (either singly or in combination), expressed either qualitatively or quantitatively.
- Estimates of the relative impact of different potential risk management measures (either singly or in combination) on risk estimates.
- Technical information on the feasibility and practicality of implementing different options.
- Benefit-cost analysis of different potential measures, including both magnitude and distribution (i.e. who benefits, who pays the costs).
- WTO SPS implications of different options in international trade situations.
Benefit-cost analysis is often difficult, even though it is a mandatory element of food safety policy decisions in some countries. Estimating the magnitude and distribution of benefits and costs of particular risk management options may require addressing such concerns as: changes in the availability or nutritional quality of foods; impacts on access to international food markets; impacts on consumer confidence in the safety of the food supply or in the food regulatory system; and other societal costs and consequences of both food safety risks and choices made in managing them. Many of these variables may be difficult to predict or quantify.
Economic estimates often have considerable uncertainty associated with them; for instance, it is difficult to predict how market participants will react to a risk-based regulation and how future markets may change. Rapid advances in science and technology add to the uncertainty in predicting benefits and costs. Thus benefit-cost analysis by itself cannot determine the best risk management choices, but as a systematic discipline for collecting and evaluating data and data gaps, it informs the decision-making process. Preferences and perceptions of those most affected by the decisions, typically, industry and consumers also need to be considered. Risk managers need to assess critically the quality of information they receive at this stage, and often must make subjective judgments as to how much weight particular considerations, and the data on which they are based, should be given.
Risk management options also often have important ethical dimensions, although they are most typically implied, rather than explicit. For example, ethical principles that underlie specific options might include the view that industry has the responsibility to provide safe food; that consumers have a right to be informed about risks associated with the foods they eat; or that government needs to act to protect those who cannot protect themselves. It may seem easier for risk managers to explain and defend food safety decisions based on scientific and economic analysis, which provide a more objective basis than ethics. But the ethical choices embedded in risk management decisions need to be openly examined to facilitate transparency and good communication.11
For examples and discussion of evaluating risk management options in two specific cases, see Annexes 2 and 3.
The process used for evaluating risk management options may vary from one risk to the next within any given country, as well as from country to country and between the national and the international levels. A desirable characteristic at all levels is an open process that provides opportunities for industry, consumers and other interested parties to provide information, to comment on proposals, and to suggest criteria for choosing preferred options. Balancing the advantages and disadvantages of multiple risk management options is already a challenging task; expanding communication with stakeholders can make this stage of the process more difficult to manage, and may lengthen the time required to complete it. Nevertheless, risk managers will find that an extensive and inclusive consultation process generally improves both the quality and the public acceptability of the ultimate decision as to the preferred risk management options.
When evaluating risk management options for microbial hazards in food, regulators should provide as much flexibility as possible in regulatory standards for the industry that is implementing them, as long as the outcome in terms of consumer protection is achieved. The HACCP system fits nicely into this flexible and outcome-driven approach. In recent years, this principle has led to the concept of risk-based targets for control of hazards at particular steps in the food production chain. Development of specific quantitative microbiological metrics – such as food safety objectives (FSOs), performance objectives (POs) and performance criteria (PCs) – that can be incorporated in regulation is discussed in Boxes 2.14 and 2.15.
Box 2.14. Codex definitions of quantitative microbiological food safety metrics*
|
Risk management options for chemical hazards in foods are often generic, such as ensuring that use of a pesticide or veterinary drug according to GAP will not result in harmful residues in food (and establishing an MRL for monitoring purposes – see next section). Where chemicals are not intentionally used in food production settings (e.g. environmental contaminants such as dioxins or methylmercury), more specific risk management options often are evaluated (e.g. imposing conditions on harvesting, providing information to consumers so that they can voluntarily limit exposure). Exposure guidelines such as Provisional Tolerable Weekly Intakes (PTWIs) (see Annex 2) can then provide a reference point for maximum safe intake, and risk management measures can be put in place that aim to prevent consumers from exceeding that safe upper limit of exposure (see next section).
Risk management options for many chemical hazards rely on approaches that estimate an acceptable exposure level for avoiding chronic adverse health effects, such as an NOAEL or RfD methodology (see Chapter 3 Risk assessment ). When other risk modelling approaches are used, such as linear modelling for carcinogenic effects, different risk management options may be identified and evaluated, such as banning or severely restricting the use of the chemical.
2.5.3. Step 3: Select a risk management option(s)
Various approaches and decision-making frameworks can be used to select risk management options (see Box 2.16). There is no one preferred approach, and different ways of reaching decisions may be appropriate for different risks and in different contexts. In essence, the risk management decision on appropriate options is arrived at by considering and integrating all of the evaluation information described above.Although there are some cases where risk reduction is not the primary objective, for example when judging the equivalence of different measures in their ability to protect human health, the foremost objective in most risk management decision-making is to reduce food-borne risks to human health. Risk managers should focus on selecting those measures that have the greatest risk-reducing impact and weigh those impacts against other factors that influence decision-making, including the feasibility and practicality of potential measures, cost-benefit considerations, stakeholder equity, ethical considerations, and creation of countervailing risks such as decreases in the availability or nutritional quality of foods.
This weighting process is essentially qualitative because of the obviously different nature of the values involved. Risk managers must decide how much weight to give each value considered. Thus the selection of the “best” risk management option is fundamentally a political and social process. Given that, the options chosen should always be in proportion to the actual public health risks involved.
| Box 2.15. Using quantitative microbiological metrics as risk management options Quantitative microbiological metrics (as defined in Box 2.14) based on risk assessments can be useful in risk management. At the international level, Codex recognizes the desirability of using POs and/or PCs as a basis for establishing practical standards, such as risk-based microbiological criteria (MC), process criteria or product criteria, but methods for doing so are still being developed. An FSO established at the point of consumption of the food provides a reference for developing microbiological targets at other points in the food production chain. One or more POs or PCs may be necessary at different stages along the chain to specify the required level of microbiological control at a particular step in food production; setting a standard on this basis (e.g. requiring a process that reduces Salmonella levels by one-million-fold when cooking ground beef) may be a risk-based regulatory option. A process criterion is a physical control measure (e.g. time, temperature) at a step, or combination of steps, that can be applied to achieve a PO. Process criteria should be validated to determine that they are achieving the required level of microbiological control on a consistent basis before being set as standards. A product criterion (pH, water activity/aw) similarly serves as a physical control measure. Process and product criteria should be risk-based to the extent possible and criteria should not be set that represent unnecessary levels of pathogen control; for instance, current processing standards for pasteurization of milk may be more severe than necessary to deliver an acceptable level of consumer protection. Methods for translating POs and PCs into risk-based MCs are still being developed. While the former specify the maximum levels of particular micro-organisms allowable in food, a risk-based MC must incorporate sampling plans of sufficient stringency that they can assure risk managers that the probability of exceeding maximum allowable limits is very low. Decisions as to where along the food production chain to apply standards based on POs (see below) may be influenced by overarching risk management goals. For example, the primary source of contamination of the food may be at the farm level (such as Campylobacter in poultry) and risk managers may be able to most effectively reduce consumer risk by setting a PO at an early point in the production chain. Alternatively, when the primary source of contamination is inadequate control at a late stage of processing (such as Listeria in cold-smoked salmon), the risk manager can exert the greatest influence on poor hygienic practice by setting a PO for a later point in the food production chain. |
2.5.3.1. Identifying a desired level of consumer health protection
The level of consumer health protection provided by a decision on risk management measures is often called the “Appropriate Level of Protection” (ALOP).12 ALOP is defined in the WTO SPS Agreement as “the level of protection deemed appropriate by the Member establishing a sanitary or phytosanitary measure to protect human, animal or plant life or health within its territory.”13 The ALOP concept is sometimes also referred to as “acceptable level of risk.” It is important to note that the ALOP is an expression of the level of protection achieved in relation to food safety at the current time. However, because the currently achieved level of consumer health protection may change (for example, new technologies may change the level of a contaminant in a food), an ALOP may be revised over time. Future objectives or goals in terms of consumer health protection may also be established. Once achieved these objectives or public health goals/targets will lead to a revision of the ALOP.
ALOPs may range from general to specific, depending upon the level of information available with regard to the source of hazards and risks. An example of a general ALOP could be the current level of Salmonella infections in a country (an example of an ALOP was the incidence of Salmonella in Finland and Sweden when they joined the European Union). An example of a specific ALOP was the background level of cryptosporidiosis in the United States as a basis for establishing levels of treatment for drinking water.
Expression of public health goals may range from the general to the specific, depending upon the level of source attribution. For example, a general public health goal would be to reduce the incidence of human Salmonella Enteritidis infections. A specific public health goal would be to reduce the incidence of human cases of Salmonella Enteritidis associated with consumption of eggs. Goals may be set either in absolute terms (e.g. number of cases per 100,000 population) or in terms of relative improvement (e.g. a percentage reduction in the number of cases).
Expression of the ALOP or a future goal with regard to the level of consumer health protection for a specific food-borne public health risk is obviously a core risk management function and, in most cases, is tied to the feasibility and practicality of available risk management options. In considering and integrating all of the evaluation information described above, a measure or measures linked to a specific level of consumer protection will be selected.
The concept of ALOP or similar future targets is essential in establishing the linkage between risk management actions and the level of consumer health protection achieved. A range of tools or approaches are available to the risk manager in bridging between practical control measures and level of consumer health protection. Some examples of these approaches are provided in Box 2.16.
For chemical contaminants, the output of the risk assessment generally includes an estimate of a tolerable intake, such as a tolerable daily intake (TDI) or PTWI (see the methylmercury case study in Annex 2 for a detailed example). For food additives, pesticide residues and residues of veterinary drugs, the risk assessors normally determine an acceptable daily intake (ADI). A TDI, PTWI or ADI is generally based on an estimate made by the risk assessors of a dose level that is reasonably certain to have no adverse health effects. It thus provides an ALOP that is pre-determined by public policy to be “notional zero risk.” A range of risk management measures that should achieve the required ALOP can be then selected for implementation; for example, enforcing GAP at farm level to minimize pesticide residues, setting MRLs for residues in specific foods, and using the MRLs to monitor the food supply.
Box 2.16. Examples of approaches to setting an Appropriate Level of Protection that are used in selecting risk management options
|
In some countries, quantitative probabilistic approaches to risk assessment of chemical hazards are changing the way decisions are made on selecting risk management options. These methods estimate changes in risks associated with changes in chemical exposure levels. A level of risk that is judged acceptable can be defined by public policy, and risk management measures can then be chosen to keep risk below that “threshold,” sometimes referred to as a “virtually safe dose.” Box 2.16 includes examples of approaches to determining an ALOP for a chemical hazard in food.
2.5.3.2. Reaching a decision on the preferred risk management option(s).
Risk managers must consider both the desired level of consumer protection and the availability and efficacy of risk management options when making this decision. Some examples have been presented in the discussion above. In general, most decision frameworks for selection of risk management options have as their primary purpose “optimization” of outcomes. That is, the decision-makers aim to achieve the “best” level of consumer protection in a manner that is as cost-effective, technically feasible, and sensitive to the rights of consumers and other stakeholders, as possible. Cost-risk-benefit analysis generally requires large amounts of information on both risks and the consequences of different risk management options. As noted, no single approach to decision-making is best for all cases, and more than one approach can be appropriate for any given food safety decision.
Box 2.17. Examples of voluntary / non-regulatory risk management measures
|
A systematic, rigorous evaluation of options, in an open process where affected parties can participate and communicate with decision-makers, is most likely to produce a sound, widely accepted decision. Given the importance of non-scientific values in the resolution of food safety problems, participation by external stakeholders is appropriate and can be critical to the successful completion of this stage. Where possible, risk management should consider the entire continuum from production to consumption, regardless of the number of authorities involved and their respective responsibilities, in order to develop the best management solutions. Any regulatory measures must be able to be enforced on the basis of the national framework of legal and regulatory authorities. However, in some countries, good results have been achieved by adopting measures that are voluntary rather than legally binding (Box 2.17). Finally, in today’s global food marketplace, regulatory measures must take into account international trade agreements and the additional obligations they impose on national authorities (see Box 2.18)
| Box 2.18. Risk management and the WTO SPS Agreement The WTO SPS Agreement sets out the basic rules for establishing safety measures for foods that are traded internationally. An SPS measure by its nature can restrict trade, for example by limiting imports of foods that do not comply with national regulations. The SPS Agreement stipulates that food safety control measures can be applied only to the extent necessary to protect human health, and should not be applied in a manner which would constitute a disguised restriction on international trade. However, some governments may, for various reasons, adopt standards that are stricter than what is required to protect health, which could be perceived as barriers to trade. Challenges to such barriers must be based on risk assessment but because of the uncertainties inherent in risk assessment and the possibility that different assessments of the same risk may yield different outcomes, and given the frequent complexity of import standards, “protectionist devices” can be difficult to identify and remove. Harmonized and transparent application of a RMF to identify and select risk management options in different countries should significantly advance the goal of preventing unjustified and unfair restrictions in the international trading of food. |
2.5.3.3. Dealing with uncertainty.
Uncertainty is an inescapable element in risk assessments and in efforts to project the impacts of risk management measures. When making risk management decisions, national food safety authorities need to take into account uncertainty, as transparently as they can. In predicting the outcome of a risk-based measure, the risk assessor should preferably use probability to express the uncertainty related to the estimate (for more discussion, see Chapter 3 Risk assessment ). From the risk manager’s perspective, uncertainty must be well enough characterized that the decisionmaker “knows when he knows enough to act”. In this context, risk managers can test their interim decisions by requesting:
- A sensitivity analysis to determine how perturbations in model inputs affect the results.
- An uncertainty analysis to determine the consequences of all the uncertainty
2.6. Implementation of the risk management decision
Risk management decisions are implemented by a variety of parties, including government officials, the food industry and consumers. The type of implementation varies according to the food safety issue, the specific circumstances and the parties involved.To effectively execute control measures, food producers and processors generally implement complete food control systems using comprehensive approaches such as GMP, GHP and HACCP systems. These approaches provide a platform for specific food safety risk management options as identified and selected by risk managers.
Industry has the primary responsibility to implement food safety controls (both regulatory and voluntary); many different national legislative arrangements provide for this allocation of food safety responsibility. Government agencies can use a variety of verification activities to ensure compliance with standards by industry. Some governments or regulatory bodies implement control measures such as physical inspection and product testing themselves, which places the primary cost of verifying compliance with standards by industry on the regulatory authority.
For some hazards, it may not be practical or cost-effective for industry to implement food control measures at each individual location at which they operate, for example testing for chemical residues of one sort or another. National chemical residue programmes can provide the data necessary to assure that appropriate control of hazards is being achieved in such circumstances. Programmes of this sort may be implemented by government, industry or both acting jointly.
In recent years, new approaches to the organization of national food safety authorities have emerged in different countries. Integrating all nationally-mandated food inspection systems under a single authority may have several advantages, such as reducing duplication of efforts and overlap of responsibilities, and improving the implementation of governmental food controls. A consolidation of multiple legislative and functional activities previously spread over several legislative jurisdictions gives practical meaning to multidisciplinary approaches to food safety and implementation of a risk-based “production-to-consumption” approach.
In parallel, food safety systems today depend increasingly on an integrated systems approach that shares responsibility for implementing food safety decisions. Innovative partnerships across the production-to-consumption continuum provide flexibility, which may be lacking in less integrated regulatory systems. For example, quality assurance systems can be extended in the case of ante- and post-mortem inspection of slaughtered animals to co-regulatory systems that include industry and veterinary service activities. For instance, in Australia, the official veterinary service is now responsible for the broad design of the inspection system and its audits and sanctions, while industry is responsible for further developing, implementing and maintaining the system. The veterinarian responsible for a specific slaughterhouse ensures that the quality assurance programme implemented by industry meets regulatory requirements on an ongoing basis.
2.7. Monitoring and review
Risk management does not end when a decision has been taken and implemented. Risk managers are responsible for verifying that the risk mitigation measures are achieving the intended results, that there are no unintended consequences associated with the measures, and that risk management goals can be sustained in the longer term. Risk management decisions should be reviewed periodically when new scientific data or insights become available, as well as when experience, such as data gathered during inspection and monitoring, warrants a review. This phase of risk management includes gathering and analysing data on human health, and on food-borne hazards that pose risks of interest, to provide an overview of food safety and consumer health.Surveillance of public health (which is a component of monitoring in a broad sense) is usually carried out by national public health authorities. It offers evidence of changes in food-borne illness rates that may follow implementation of risk management measures, as well as the potential for identifying new food safety problems as they emerge. When surveillance yields evidence that required food safety goals are not being achieved, redesign of food safety controls by government and industry is needed.
Box 2.19 illustrates some kinds of information that are useful for monitoring the effects of risk management measures.
Box 2.19. Examples of information that can be used for monitoring the effects of risk management measures
|
Most food safety authorities apply regulatory programmes at various points in the food production chain to monitor the presence of specific hazards; for example, national residue surveys, national monitoring programmes for microbial pathogens in fresh meat. Even though these programmes may not be integrated into an overall food control system, they provide valuable information on the changing prevalence of hazards over time and the level of regulatory compliance.
Human health surveillance to complete the RMF process is ordinarily outside of the jurisdiction of many food safety authorities but may be a responsibility of an overarching government authority. Monitoring and review activities should be specifically designed to support management of food-borne risks and provide the opportunity for multidisciplinary inputs in a risk-based food safety system. Food-borne disease investigations, analytical epidemiological studies such as food source attribution, case-control investigations and strain typing of bacterial hazards to genotype level can provide a valuable adjunct to human health surveillance.
In some cases, monitoring might result in a request for a new risk assessment, perhaps reducing previous uncertainties, or updating the analysis with new or additional research findings. Revised risk assessment results could lead to reiteration of the risk management process, with possible changes in risk management goals and the risk management option chosen. Changes in broad-based public health goals, changing societal values and technological innovations all can provide reasons to revisit risk management decisions previously taken.
2.8. Suggestions for further reading
FAO/WHO. 1997. Risk management and food safety. Report of a Joint FAO/WHO Consultation. Rome, Italy, 27-31 January 1997. FAO Food and Nutrition Paper No. 65 (available at: http://www.fao.org/docrep/W4982E/w4982e00.htm).FAO/WHO. 1999. The application of risk communication to food standards and safety matters. Report of a Joint FAO/WHO Expert Consultation. Rome, 2-6 February 1998. FAO Food and Nutrition Paper No. 70 (available at: http://www.fao.org/docrep/005/x1271e/x1271e00.htm).
FAO/WHO. 2002. Principles and guidelines for incorporating microbiological risk assessment in the development of food safety standards, guidelines and related texts.
Report of a Joint FAO/WHO Consultation. Kiel, Germany, 18-22 March 2002 (available at: ftp://ftp.fao.org/docrep/fao/006/y4302e/y4302e00.pdf).
FAO. 2004. Food Safety: Science and Ethics. Report of an Expert Consultation. Rome, Italy, 3-5 September 2002. FAO Readings in Ethics (available at ftp://ftp.fao.org/docrep/fao/006/j0776e/j0776e00.pdf).
FAO/WHO. 2005. Report of the 37th Session of the Codex Committee on Food Hygiene. Buenos Aires, Argentina, 14-19 March 2005. ALINORM 05/28/13 (available at: http://www.codexalimentarius.net/web/archives.jsp?year=05).
FAO/WHO. 2006. The Use of Microbiological Risk Assessment Outputs to develop Practical Risk management Strategies: Metrics to improve food safety. Report of a Joint FAO/WHO Meeting in collaboration with the German Federal Ministry of Food, Agriculture and Consumer Protection. Kiel, Germany, 3-7 April 2006 (available at: http://www.fao.org/ag/agn/jemra/index_en.stm).
Morgan, G. and Henrion, M., eds. 1992. Uncertainty: A guide to dealing with uncertainty in quantitative risk and policy analysis. Cambridge University Press, New York
Notes:
5
For the purposes of this Guide, risk managers are generally assumed to be officials of a national food safety authority (also called the “Competent Authority” in language of the SPS Agreement). In practice, managers in industry and many other officials can also serve as risk managers.
6
FAO/WHO. 2005. Proposed draft principles and guidelines for the conduct of microbiological risk management. Appendix III In Report of the 37th Session of the Codex Committee on Food Hygiene. Buenos Aires, Argentina, 14-19 March 2005. ALINORM 05/28/13. Codex Committee on Food Hygiene (available at: ftp://ftp.fao.org/codex/ccfh37/fh37_06e.pdf and http://www.codexalimentarius.net/web/archives.jsp?year=05).
7
Preliminary risk management activities were referred to as “risk evaluation” in the past. In the 13th Edition of the Codex Procedural Manual, “risk evaluation” was defined as a “preliminary risk management activity” to differentiate it from “risk assessment.”
8
FAO. 2003. Food Safety: Science and Ethics. Report of an Expert Consultation. Rome, Italy, 3-5 September 2002. FAO Readings in Ethics 1 (available at: http://www.fao.org/documents/show_cdr.asp?url_file=/DOCREP/006/j0776e/j0776e08.htm).
9
Information about risk assessments carried out by JECFA, JEMRA and JMPR is available on the Internet. JECFA: www.fao.org/ag/agn/jecfa/index_en.stm and www.who.int/ipcs/publications/jecfa/en/index.html; JEMRA: www.fao.org/ag/agn/jemra/index_en.stm and www.who.int/foodsafety/micro/jemra/en/index.html; and JMPR: www.fao.org/ag/agp/agpp/pesticid/ and http://www.who.int/ipcs/publications/jmpr/en/
10
In cases where risk management is focused on a single hazard, this step will not apply.
11
FAO. 2003. Food Safety: Science and Ethics. Report of an Expert Consultation. Rome, Italy, 3-5 September 2002. FAO Readings in Ethics 1 (available at: http://www.fao.org/documents/show_cdr.asp?url_file=/DOCREP/006/j0776e/j0776e08.htm).
12
See Annex 5 (Introducing the WTO SPS and TBT Agreements) In FAO. 2003. Assuring food safety and quality. Guidelines for strengthening national food control systems. FAO Food and Nutrition Paper No. 76 (available at: ftp://ftp.fao.org/docrep/fao/006/y8705e/y8705e00.pdf).
13
FAO/WHO. 2000. The Interaction between assessors and managers of microbiological hazards in food. Report of a WHO Expert Consultation in collaboration with the Institute for Hygiene and Food Safety of the Federal Dairy Research Centre, Germany and the Food and Agriculture Organization of the United Nations (FAO). Kiel, Germany, 21-23 March 2000.
Source: FAO
 naar boven
naar boven



 bedrijven
bedrijven
