Dieser Artikel ist noch nicht auf Deutsch vorhanden
Oxygen Operating Principles
Inhalt
 | Relation partial pressure - % oxygen | |
 | Percent saturation | |
 | Concentration versus temperature | |
 | Salinity |
The physical properties of oxygen are related to temperature, so that a thermistor is often fitted in the probe to provide temperature compensation. A constant voltage is placed across the cathode and anode. Molecular oxygen diffuses through the membrane and is reduced at the cathode by the applied voltage. This electrical process results in a current flow. The instrument detects this current which is proportional to the partial pressure of oxygen. The ideal gas law expresses the proportional relationship between gas pressure P and n, the number of gas molecules present.
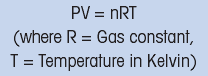
This relationship, and the ability of the meter to compensate for temperature changes, allows results to be expressed as percentage oxygen or mg / l O2.
Relation partial pressure - % oxygen
Air is a mixture of gases in wich oxygen contributes 21% of the total pressure. Consider a volume of air at atmospheric pressure, 760 mm Hg. 21% of this pressure (160mm Hg) is contributed by oxygen.If the total pressure on this system is doubled to 1520 mm Hg, the partial pressure also doubles to 320 mm Hg. The relative percent of the oxygen is still 21%. However, the sensor and instrument would see a two-fold increase in the oxygen concentration since the sensor is only responding to the partial pressure of oxygen (Fig. 1).
For this reason, air calibration of this system in the percent oxygen mode is valide only only if the gas mixture is under a total pressure of one atmosphere. Sample correction can be made to convert oxygen partial pressure values to percent oxygen at other pressures as shown in the table (Fig. 2).
Gases are soluble in liquid to varying degrees. This solubility, expressed as a fraction mole, is proportional to the partial pressure of the gas over the liquid (Henry’s law). For most dissolved oxygen applications, the desired units are parts per million, ppm (when liquid density is 1 g / cm3, ppm equals mg / I O2). The fraction mole is easily converted to these units.
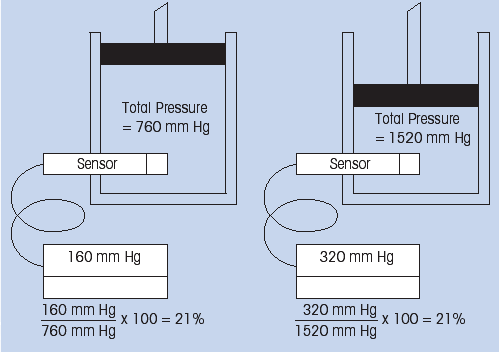
(Fig. 1: Relationship between partial pressure and % oxygen)

(Fig. 2: Formula for sample correction)
Percent saturation
Percent saturation is another parameter that is frequently used. This is the amount of oxygen the liquid can hold at that temperature. For a given liquid, a constant relationship exsist between percent saturation and concentration at various temperatures. This relationship must be determined experimentally.To illustrate this relationship, consider a container of 100% air satured water. As the container is heated and the water begins to boil, bubbles of air form in the container. These bubbles would eventually rise to the surface and then leave the liquid.
One would intuitively conclude that the concentration of air in the solution has decreased, however, the water would still be 100% satured with air at that temperature (Fig. 3).
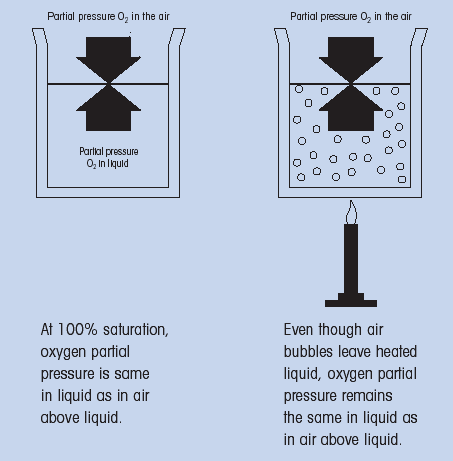
(Fig. 3: Relationship between % saturation and concentration)
Concentration versus temperature
PPM dissolved oxygen versus temperatureThis phenomenon of decreasing solubility of gases at increasing temperature (Fig. 4) can be accounted for by the thermodynamic relationship between free energy and entropy. If the temperature was held constant, than the relationship between percent saturation and concentration (ppm) could be established. It can be seen that percent saturation is linearly proportional to concentration (Fig. 5). As mentioned before, the exact relationship between percent saturation and concentration must be determined empirically for each different sample
Salinity
The ionic strenght (salt content) of natural and waste waters has a considerable effect on dissolved oxygen levels. In order to obtain dissolved oxygen results, correction for saltingout effects have to be made. Such effects greatly reduce electrode sensitivity.Salinity has been defined traditionally as the total solids in water after all carbonates have been converted to oxides, all bromide and iodide to chloride and all organic compounds oxidized. However, for practical purposes, the definition of salinity is based upon the conductivity of seawater relative to a specified potassium chloride solution. Therefore the measurement of solution conductivity greatly simplifies the calibration and usage of dissolved oxygen measuring systems.
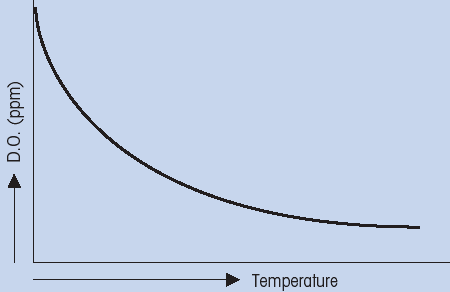
(Fig. 4: Relationship between dissolved oxygen and temperature)
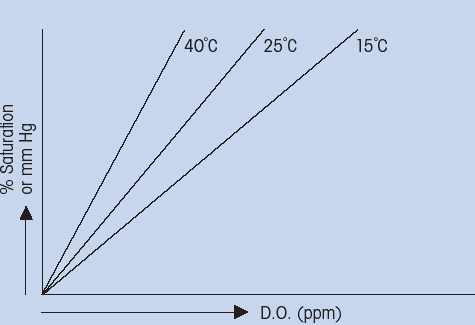
(Fig. 5: Temperature dependence of relationship between % saturation and concentration)
 Nach oben
Nach oben


 Firmen
Firmen