This article is not yet available in the language you selected
3 Risk assessment
Article index
Summary
Risk assessment is the scientific foundation of risk analysis. This chapter takes a broad view of risk assessment methodologies and their essential characteristics. The four steps in the Codex risk assessment system are fully explored, together with risk ranking and epidemiological approaches. The responsibilities of risk managers in commissioning and administering a risk assessment are described and differences between risk assessment approaches for chemical compared with microbiological hazards are illustrated. The relative merits of qualitative and quantitative approaches are examined, as are recent approaches using probabilistic models of risks.3.1. Introduction
Risk assessment is the central scientific component of risk analysis and has evolved primarily because of the need to make decisions to protect health in the face of scientific uncertainty. Risk assessment can be generally described as characterizing the potential adverse effects to life and health resulting from exposure to hazards over a specified time period.Risk management and risk assessment are separate but closely linked activities, and ongoing, effective communication between those carrying out the separate functions is essential. As described in Chapter 2 Risk management, risk managers applying the RMF must decide whether a risk assessment is possible and necessary. If this decision is affirmative, risk managers commission and manage the risk assessment, carrying out tasks such as describing the purpose of risk assessment and the food safety questions to be answered, establishing risk assessment policy, setting time schedules and providing the resources necessary to carry out the work.
This chapter describes the substantive content of the food safety risk assessment process and explains how risk assessment fits into application of the RMF. While the main focus is on application of risk assessment methodology as defined by Codex (i.e. systematic application of the four steps listed in "Introduction to risk analysis" section 1.2.1), a broader view of risk assessment is also taken. All methods for assessing risks described here use the best scientific knowledge available to support risk-based standards or other risk management options.
and this probably is their greatest strength. Scientific approaches that combine risk assessment, epidemiology14 and economics are likely to be most useful to risk managers trying to integrate and balance risks and benefits.
3.1.1. Risk assessment and the WTO SPS Agreement
WTO members are bound by the provisions of the SPS Agreement, which places risk assessment within a coherent SPS system for developing and applying standards for food in international trade. The scope of the SPS Agreement in the context of this Guide covers risks to human life and health, and requires that WTO members:- Shall ensure that any measure is applied only to the extent necessary to protect human life and health.
- Shall base their measures on risk assessment, taking into account the techniques developed by the relevant international organizations.
- May implement a measure that differs from international norms where a higher “appropriate level of health protection” is a legitimate goal.
- Shall apply the principles of equivalency where a different measure in an exporting country achieves their appropriate level of protection.
3.1.2. Relative positions of risk assessment and risk management
The place occupied by risk assessment during an application of the RMF by risk managers is described in Chapter 2 "An introduction to risk analysis". Although risk managers commission and guide the production of a risk assessment and evaluate its outputs, the risk assessment itself is generally an external product, independently produced by scientists.3.2. Scientific approaches for assessing risks
When addressing a particular food safety issue, an early risk management decision concerns the scientific approach that will be taken (see Risk management, section 2.4.1, Step 3). While this chapter is focused on risk assessment as an input to the RMF, there are many situations at the national level where no risk assessment of any form is available or feasible. In other situations, an active decision may be taken to use a scientific approach that does not include risk assessment. Obviously the advantages that flow from using risk assessment to set food safety control measures (see Chapter 2 Risk management ) cannot be realized in such scenarios; nevertheless, choices to apply other scientific approaches are likely to be reasonable and appropriate in their own right.This Guide takes the broad view that several approaches to risk assessment can be used to establish an association of sufficient strength between food-borne hazards, control measures and risks to consumers, such that controls can be genuinely described as “risk-based” (see "An introduction to risk analysis"). Often, a combination of approaches may contribute to the risk assessment as a whole. This perspective shifts the focus from prescription of risk assessment methodology (as in Codex) to the outcome, and encourages food regulators to use methods best suited to the task. Where resources are limited, this Guide also may provide regulators with simpler methods that still lead to standards that can reasonably be described as risk-based, i.e. based on a scientific assessment of risk. Recognition that a range of approaches can lead to a risk-based standard also brings flexibility to the issue of the level of risk assessment rigor needed in low-risk situations.
In promulgating a flexible approach to use of risk assessment methodology, this Guide advocates that the RMF process should always include a risk profile of some sort. In applying the RMF, risk managers may directly use the information in the risk profile to identify and select food standards. Box 3.1 and Box 3.2 present examples illustrating the direct use of a risk profile as a basis for risk management decisions in cases where it was either unnecessary or not feasible to carry out a risk assessment. While basing risk management decisions on a risk profile may be fully justifiable in particular circumstances, the resulting standards are not ordinarily considered to be risk-based.
Box 3.1. Examples of direct use of a risk profile to establish food safety standards
|
3.2.1. Risk assessment
Risk assessment incorporating, in one way or another, the four analytical steps described by Codex (see Figure 3.1) is the main focus of this chapter. The way those steps are applied differs somewhat for microbiological and chemical hazards.Figure 3.1. Generic Codex description of the components of risk assessment
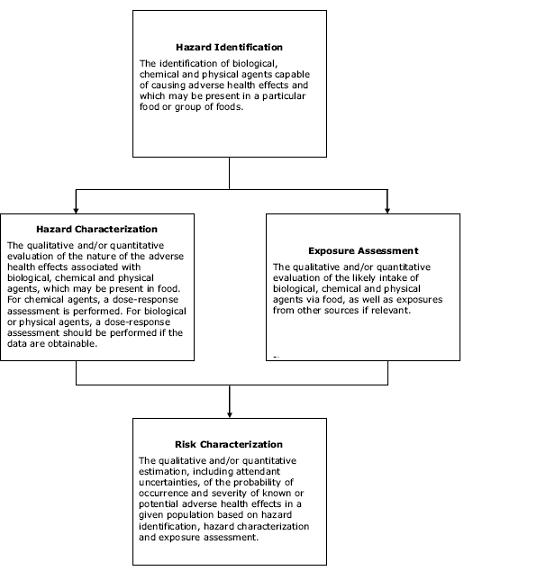
For microbiological hazards, the occurrence and transmission of the hazard at various stages from food production to consumption is evaluated, thus moving “forward” through the various stages of the food chain to arrive at an estimate of risk. While the accuracy of estimated risks is often limited by uncertain dose-response information, the greatest strength of such risk assessments arguably lies in their ability to model the relative impacts of different food control measures on risk estimates.
| Box 3.2. The Canadian approach to regulating Listeria monocytogenes in ready-to-eat foods When the Canadian government did a risk profile of this problem they recognized that contamination by L. monocytogenes could be reduced, but not eliminated from the final product or the environment. Risk management policy focuses inspection, testing and compliance action on ready-to-eat foods that are capable of supporting growth of L. monocytogenes. Specific attention is paid to those foods that have been linked to food-borne illness, and those with more than a ten day shelf life. In this approach, ready-to-eat foods are placed in one of three categories:
|
In contrast, for chemical hazards, “safety evaluation” is a standard risk assessment methodology16. In that approach, maximum exposure levels are identified to fit a “notional zero risk” outcome (a dose level that is reasonably certain to pose no appreciable risk to the consumer). This approach does not produce precise estimates of risk versus dose and cannot model the impact of various interventions in terms of risk reduction. These differences are explored further in section 3.5.
3.2.2. Use of ranking tools
Risk ranking, using tools that rely on knowledge of risk factors to rank risks and prioritize regulatory controls, is often commissioned by risk managers (Box 3.3). Such rankings may or may not be based on risk assessments. Some tools categorize a food business against specified risk factors, e.g. by type of food, type of food preparation, type of business, complianceBox 3.3. Examples of risk ranking tools
|
record, food user subpopulation. Other tools are used to rank hazard-food combinations in a national context by deriving a “comparative risk” scoring system. While risk ranking methods not based on risk assessments assist risk-based food regulation, their use of scoring systems (which inevitably have subjective, arbitrary elements) to derive regulatory standards has inherent shortcomings. Thus they are not a good substitute for ranking methodologies that do incorporate risk assessment.
3.2.3. Epidemiology
Epidemiology is increasingly being used in food safety to study the links between the frequency and distribution of adverse health effects in specific populations and specific food-borne hazards. This includes observational studies of human illness such as case-control, analysis of surveillance data, and focused research. The usefulness of epidemiology depends on the availability of data.Epidemiology is probably the most reliable approach to assess the current burden of illness, follow trends over time and attribute risks to sources. It is an important source of information for risk assessment, particularly the hazard identification and hazard characterization steps. As a stand-alone tool, epidemiology uses human illness data and works “backwards” to attribute risks and risk factors to foods; therefore it cannot generally be used to investigate the effects of different food safety control measures in reducing risk. However, risk assessment incorporating epidemiological data can be used to evaluate the impact of various changes or interventions in the food chain in terms of reducing risks. In other words, the risk assessment approach works forward from the relevant points in the food chain to estimate the risk to human health normally associated with a particular hazard-food combination.
Box 3.4. Examples of food source attribution supporting the development of risk-based standards for microbiological hazards in foods
|
Food source attribution is particularly valuable in food safety risk management (see Box 3.4). Risk assessments often address only a single hazard or, in the microbiological field, a single hazard-food combination, whereas at some stage risk managers need to have good scientific information on all transmission pathways and their relative contributions to the aggregate risk from the hazard. Risk assessments can be designed to answer this question (see example in Annex 3), but other food source attribution approaches are more commonly used, such as analysis of outbreak data, or genotyping of human microbial isolates from multiple outbreak situations where it is known that some genotypes occur predominantly in a single animal reservoir or food type. However, food source attribution often proves difficult as sporadic cases of illness are rarely represented in the available surveillance data and these may collectively cause many more cases than the outbreaks that are primarily recorded.
The use of analytical epidemiology to support development of risk-based standards depends on the availability of sufficient surveillance data on food-borne illness. Many governments are currently strengthening surveillance systems so they can better apply analytical epidemiological techniques, as well as validate microbiological risk assessment models. A detailed description of the application of epidemiological techniques is beyond the scope of this chapter.
3.2.4. Combinations of approaches
Distinctions are drawn in this chapter between risk assessment approaches based on the four analytical steps described by Codex, the use of ranking tools and the use of analytical epidemiological techniques. However, as a practical matter these various approaches are often used in combination or feed into each other (e.g. epidemiological data feed into hazard identification and hazard characterization steps of any risk assessment). Ways in which they can be integrated vary widely on a case-by-case basis, but all are subject to the general principles and guidelines described in the sections that follow.The remainder of this chapter is focused on risk assessment conducted according to the Codex methodology.
3.3. Responsibilities of risk managers in commissioning & administering a risk assessment
The decision to proceed with a risk assessment depends on factors such as the health risk priority ranking, urgency, regulatory needs and availability of resources and data.It is likely that a risk assessment will not be commissioned when:
- The risk is well described by definitive data.
- The food safety issue is relatively simple.
- The food safety issue is not of regulatory concern or not subject to regulatory mandate.
- An urgent regulatory response is required.
- The hazard exposure pathway is complex.
- Data on the hazard(s) and/or health impacts are incomplete.
- The issue is of significant regulatory and/or stakeholder concern.
- There is a mandatory regulatory requirement for a risk assessment.
- There is a need to verify that an interim (or precautionary) regulatory response to an urgent food safety problem is scientifically justified.
Box 3.5. General responsibilities of risk managers in commissioning and administering a risk assessment
|
Risk managers, in consultation with risk assessors, should fulfil several tasks when commissioning a risk assessment and seeing it through to completion (Box 3.5). While risk managers do not need to know all the details of how a risk assessment is carried out, they do need a general understanding of risk assessment methodologies and what the outcomes mean.
This understanding is both acquired through, and contributes to, successful risk communication (see Chapter 4 Risk communication).
3.3.1. Forming the risk assessment team
A risk assessment team should be appropriate to the circumstances. When strategic and large-scale risk assessments are undertaken, the general criteria described below relating to risk assessment teams apply. However, small-scale and straightforward risk assessments may be undertaken by very small teams or even by individuals, especially where a primary risk assessment is already available and the scientific work involves mostly adaptation using local data.A large-scale risk assessment generally requires a multidisciplinary team that may include experts with biological, chemical, food technology, epidemiological, medical, statistical and modelling skills, among others. Finding scientists with the required knowledge and expertise can be a challenging task for risk managers. Where government food safety agencies do not have a large scientific staff of their own upon which to draw, risk assessors are generally recruited from the national scientific community. In some countries, national science academies may organize expert committees to carry out risk assessments for the government, and private companies that conduct risk assessments on a contract basis are also becoming more widespread.
Risk managers need to take care to ensure that the assembled team is objective, balanced in terms of scientific perspectives, and free from undue biases and conflicts of interest. It is also crucial to elicit information about potential financial or personal conflicts of interest that could bias an individual’s scientific judgement. Typically, this information is solicited by a questionnaire before appointments are made to a risk assessment team. Exceptions are sometimes made if an individual has essential, unique expertise; transparency is essential when any such decisions on inclusion are made. The FAO/WHO framework for the provision of scientific advice on food safety and nutrition may provide guidance in this area17.
3.3.2. Specification of purpose and scope
Risk managers should prepare a “purpose statement” for a risk assessment, which should identify the specific risk or risks to be estimated and the broad risk management goal(s). For example, a risk assessment might be designed to provide quantitative estimates of food-borne risks due to Campylobacter in broiler chickens on an annual basis for the national population, and the risk assessment might be primarily used to evaluate risk management options at various points from production to consumption of broiler chickens, to maximize reduction in risk. The purpose statement generally flows directly from the risk management goal(s) agreed on when the risk assessment is commissioned (see Chapter 2 Risk management, section 2.4.3).In some situations, an initial exercise may be to set up a risk assessment framework model, to identify data gaps and establish the research programme required to generate the scientific inputs needed to complete a risk assessment at a later date. Where a risk assessment can be completed using currently available scientific knowledge, the model can still identify further research that will allow later refinement of the outputs.
The “scope” portion of the risk assessment description should identify the parts of the food production chain that are to be evaluated and should establish boundaries for risk assessors with regard to the nature and extent of scientific information to be considered. Risk managers addressing specific food safety issues at the national level should also be aware of international risk assessments and other pre-existing scientific efforts on relevant subjects before they commission new work (see Chapter 1, section 1.2.3, and Chapter 2, section 2.4.6). By considering existing risk assessments in consultation with their risk assessors, risk managers may be able to substantially narrow the scope of the work and the data needed.
3.3.3. Questions to be addressed by risk assessors
Risk managers, in consultation with risk assessors, should formulate the specific questions that need to be answered by the risk assessment. Depending on the scope of the risk assessment needed and the resources available, considerable discussion may be required to arrive at clear and realizable questions which will yield answers to guide risk management decisions. As with the statement on purpose and scope, questions to be addressed by the risk assessment often flow from the broad risk management goal(s) agreed on when the risk assessment is commissioned. Examples of questions that risk managers might ask risk assessors to answer are illustrated in Box 3.6. The questions asked by the risk managers can have an important influence on the choice of risk assessment methodologies used to answer them.| Box 3.6. Examples of questions to be addressed by risk assessors In the example of Campylobacter in broiler chickens used in section 3.3.2, risk assessors could be asked to address any of the following questions:
|
3.3.4. Establishing risk assessment policy
While risk assessment is fundamentally an objective, scientific activity, it inevitably contains some elements of policy and subjective scientific judgement. For example, when scientific uncertainty is encountered in the risk assessment, inferential bridges are needed to allow the process to continue. The judgements made by the scientists or risk assessors often entail a choice among several scientifically plausible options, and policy considerations inevitably affect, and perhaps determine, some of the choices. Thus gaps in scientific knowledge are| Box 3.7. Examples of choices that might be part of a risk assessment policy Policies governing values-based choices:
|
bridged through a set of inferences and “default assumptions.” At other points in a risk assessment, assumptions may be required that are driven by values-based, social consensus, often developed through long experience with how such issues should be handled. Box 3.7 presents some examples of each of these types of choices that might arise in a food safety risk assessment.
Documentation of all such default assumptions contributes to the consistency and transparency of risk assessments. These policy decisions are spelled out in a risk assessment policy, which should be developed by risk managers and risk assessors in active collaboration in advance of the risk assessment. Policies governing values-based choices and judgements should be decided primarily by risk managers (see Chapter 2), whereas policies governing science-based choices and judgements should be decided primarily by risk assessors, with active communication between the two functional groups in each case.
Pre-determining risk assessment policy for scientific aspects of a risk assessment is especially difficult when it concerns sufficiency of scientific evidence. Often, only limited data sets are available at a particular step and scientific judgements are required if risk assessment is to proceed. While risk assessment policy in a broad sense may be able to guide these judgements, they are more likely to be made on a “case-by-case” basis. Different national legal contexts also influence the way sufficiency of evidence and scientific uncertainty are addressed.
3.3.5. Specification of form of the outputs
Outputs of a risk assessment may be sought in non-numerical (qualitative) or numerical (quantitative) form. Non-numerical risk estimates provide a less definitive basis for decisions but are adequate for several purposes, such as establishing relative risks or evaluating relative impacts on risk reduction of different control measures. Numeric estimates of risk can take one of two formats:- Point estimate, which is a single numerical value representing for example the risk in a worst case scenario.
- Probabilistic risk estimates, which include variability and uncertainty and are presented as a distribution reflecting more real-life situations (see section 3.4.5).
3.3.6. Time and resources
While it is desirable to maximize scientific inputs and commission specific research to fill data gaps when conducting a risk assessment, all risk assessments are inevitably constrained in some ways. In commissioning a risk assessment, risk managers must ensure that sufficient resources (e.g. time, money, personnel and expertise) are available relative to the purpose and scope, and establish a realistic timetable for completion of the work.3.4. General characteristics of risk assessment
Irrespective of the context, risk assessments generally share a number of basic characteristics (Box 3.8). While these attributes are described comprehensively in the sections that follow, in some situations a specific risk assessment is a relatively simple and straightforward exercise. In such cases, the general characteristics can be substantially modified; for instance, it may sometimes be possible for experts within a government food safety agency to conduct an adequate risk assessment quickly and efficiently, without the need to assemble a multidisciplinary risk assessment team.Box 3.8. General characteristics of food safety risk assessments
|
3.4.1. Objectivity and transparency
A risk assessment should be objective and unbiased. Opinions or value judgements on issues other than science (for instance on economic, political, legal or environmental aspects of the risk) should not be allowed to influence the outcome and risk assessors should explicitly identify and discuss any judgements on the sufficiency of the science that was relied on.A participatory process should be used in initiating, performing and finalising a risk assessment and reporting should be in a style that allows risk managers and other stakeholders to properly understand the process. Above all, a risk assessment must be transparent and in documenting the process the risk managers should:
- Describe the scientific rationale.
- Reveal any biases that may affect the conduct or results of the risk assessment.
- Identify clearly and concisely all scientific inputs.
- Clearly state all assumptions.
- Provide an interpretive summary for lay readers.
- Where possible, make assessments available to the public for comment.
3.4.2. Functional separation of risk assessment and risk management
In general, the functions of risk assessment and risk management should be carried out separately to the extent practicable, so that the science remains independent from regulatory policy and values. However, delineating the functional boundaries between risk assessors, risk managers and risk communicators in all situations is a significant challenge. Functional separation may be more obvious when different bodies or officials are responsible for risk assessment and risk management tasks. However, functional separation can also be achieved in countries with limited resources and personnel where risk assessments are undertaken by people who act as both risk assessors and risk managers. What is important in these cases is to have conditions in place which ensure that risk assessment tasks are carried out separately from risk management tasks (see section 2.4.6). In such cases, particular attention should be devoted to ensuring that the risk assessment meets the criteria laid out in Box 3.8. Whatever the functional separation arrangements, a highly interactive, iterative process is essential for risk analysis as a whole to be effective. Communication between risk assessors and risk managers is also a critical element in the process, as described in more detail in Chapter 4 Risk communication.3.4.3. Structured process
Risk assessments should follow a structured and systematic process; see section 3.5 on risk assessment methodology.3.4.4. Basis in science
It is a primary tenet that risk assessment be soundly based on scientific data. Data of sufficient quality, detail and representativeness must be located from appropriate sources and assembled in a systematic manner. Descriptive and computational elements should be supported with scientific references and accepted scientific methodologies, as appropriate.When a risk assessment is commissioned, there often are insufficient data available to complete the assignment. Scientific information to support many food safety risk assessments is available from a variety of sources, both national and international (Box 3.9). Risk assessments carried out at the national level are rapidly increasing in number and many of them can be accessed through web-based portals. For instance, microbiological risk assessments carried out by the United States Food Safety and Inspection Service are available at www.fsis.usda.gov/Science/Risk_Assessments/index.asp.
FAO and WHO administer international panels of experts on chemical (JECFA and JMPR) and microbiological hazards (JEMRA) to provide risk assessments as the basis for Codex standards. These assessments are also used by risk assessors and risk managers at the national level.
Box 3.9. Sources of scientific information for risk assessments
|
While risk assessors conducting a given risk assessment may try to fill data gaps and to obtain adequate input data, inevitably default assumptions will need to be made at some steps during risk assessment. These assumptions must remain as objective, biologically realistic and consistent as possible. Risk assessment policy provides broad guidelines but default assumptions specific to a particular problem may have to be made on a case-by-case basis. It is essential that any such assumptions are transparently documented.
Sometimes when data are lacking, expert opinions can be used to address important questions and uncertainties. A variety of knowledge elicitation techniques have been developed for this purpose. Experts may be unaccustomed to describing what they know or how they know it; knowledge elicitation techniques reveal expert knowledge and help to make expert opinions as evidence-based as possible. Approaches that can be used include interviews, the Delphi method18, surveys and questionnaires, among others.
3.4.5. Dealing with uncertainty and variability
Definitive data needed to derive quantitative risk estimates are often lacking, and sometimes there are significant uncertainties inherent in biological or other models used to represent the processes that contribute to risk. Uncertainty about the available scientific information is often addressed in a risk assessment by using a range of possible data values.Box 3.10. Examples of uncertainty and variability in risk assessments
|
Two distinct characteristics of scientific information are relevant in this context. Variability is a characteristic of phenomena that differ from one observation to the next; for example, people eat different amounts of a food, and the level of a particular hazard present in a food also can vary widely from one serving of food to another. Uncertainty is the quality of being unknown, for example because inadequate data exist, or because the biological phenomena involved are not well understood. For instance, in assessing a chemical hazard scientists may need to rely on data from toxicity tests in rodents because insufficient human epidemiological data exist. For examples of each kind of uncertainty, see Box 3.10.
Risk assessors must ensure that risk managers understand the impacts of limitations of available data on the results of the risk assessment. Risk assessors should provide an explicit description of uncertainties in the risk estimate and their origins. The risk assessment should also describe how default assumptions may have influenced the degree of uncertainty in the outputs. As necessary or appropriate, the degree of uncertainty in the results of a risk assessment should be described separately from the effects of variability inherent in any biological system.
Deterministic chemical risk assessments (see section 3.5.2.1) for chronic adverse health effects use point estimates to represent data and typically do not explicitly quantify uncertainty or variability in outcomes (see section 3.5).
3.4.6. Peer review
Peer review reinforces transparency and allows wider scientific opinion to be canvassed in relation to a specific food safety issue. External review is especially important where new scientific approaches are being applied. Open comparison of the outcomes of similar risk assessments where different scientific defaults and other judgements have been used can yield useful insights.3.5. Risk assessment methodology
Different risk assessment methods are used in different countries and within countries, and different methods may be used to assess different kinds of food safety problems. Methods vary according to the class of hazard (i.e. chemical, biological or physical hazard), the food safety scenario (e.g. concerning known hazards, emerging hazards, new technologies such as biotechnology, complex hazard pathways such as for antimicrobial resistance) and the time and resources available. This section provides only a brief overview of methods; readers who wish to gain deeper understanding can consult the references listed at the end of the chapter.Differences in risk assessment methodology are most apparent for chemical compared with microbiological hazards. This is partly due to intrinsic differences between the two classes of hazards (Box 3.11). The differences also reflect the fact that for many chemical hazards, a choice can be made as to how much of the chemical may enter the food supply, e.g. for food additives, residues of veterinary drugs and pesticides used on crops. Use of these chemicals can be regulated or restricted so that residues at the point of consumption do not result in risks to human health. Microbial hazards, in contrast, are ubiquitous in the food chain, they grow and die, and despite control efforts, they often can exist at the point of consumption at levels that do present obvious risks to human health
| Box 3.11. Some characteristics of microbial and chemical hazards that influence the choice of risk assessment methodology | |
|
Microbial Hazard
|
Chemical Hazard
|
3.5.1. Basic components of a risk assessment
The risk assessment process is generally represented as consisting of four steps, described by Codex (see Figure 3.1 in section 3.2.1 above). Following identification of the hazard(s), the order in which these tasks can be carried out is not fixed; the process is normally highly iterative, with steps repeated as data and assumptions are refined.3.5.1.1. Hazard identification
Specific identification of the hazard(s) of concern is a key step in risk assessment and begins a process of estimation of risks specifically due to that hazard(s). Hazard identification may have already been carried out to a sufficient level during risk profiling (see Chapter 2); this generally is the case for risks due to chemical hazards. For microbial hazards, the risk profile may have identified specific risk factors associated with different strains of pathogens, and subsequent risk assessment may focus on particular subtypes. Risk managers are the primary arbiters of such decisions.
3.5.1.2. Hazard characterization
During hazard characterization, risk assessors describe the nature and extent of the adverse health effects known to be associated with the specific hazard. If possible, a dose-response relationship is established between different levels of exposure to the hazard in food at the point of consumption and the likelihood of different adverse health effects. Types of data that can be used to establish dose-response relationships include animal toxicity studies, clinical human exposure studies and epidemiological data from investigations of illness.
Response parameters may be categorized according to the risk management questions that are asked of risk assessors; for example, for chemical hazards, type of adverse health effects induced by different doses of chemical hazards in animal tests; for microbial hazards, infection, morbidity, hospitalization and death rates associated with different doses. Where economic analyses are undertaken, hazard characterization should include the large impact of food-borne illness that is due to complications following the acute phase, e.g. with haemolytic uraemic syndrome with E. coli O157:H7, and with Guillain-Barré syndrome with Campylobacter.
3.5.1.3. Exposure assessment
Exposure assessment characterizes the amount of hazard that is consumed by various members of the exposed population(s). The analysis makes use of the levels of hazard in raw materials, in food ingredients added to the primary food and in the general food environment to track changes in levels throughout the food production chain. These data are combined with the food consumption patterns of the target consumer population to assess exposure to the hazard over a particular period of time in foods as actually consumed.
Characterization of exposure may vary according to whether the focus is on acute or chronic adverse health effects. Risks from chemical hazards are typically assessed against long-term or lifetime chronic exposure to the hazard, often from multiple sources. Acute exposures are also frequently considered for certain contaminants and pesticide and veterinary drug residues. Risks from microbial hazards are typically evaluated in terms of single exposures to a contaminated food.
The level of a hazard in a food at the time of consumption is often very different from that when the food is being produced. Where necessary, exposure assessment can scientifically evaluate changes in levels of hazard throughout the production process to estimate the likely level at the time of consumption. In the case of chemical hazards in foods, there may be relatively little change from levels in raw materials. In the case of microbiological hazards in foods, marked changes in levels can occur due to pathogen growth, and cross-contamination at the time of final preparation for consumption may add to the complexity of the evaluation.
3.5.1.4. Risk characterization
During risk characterization, outputs from the previous three steps are integrated to generate an estimate of risk. Estimates can take a number of forms and uncertainty and variability must also be described if possible (see section 3.4.5). A risk characterization often includes narrative on other aspects of the risk assessment, such as comparative rankings with risks from other foods, impacts on risk of various “what if” scenarios, and further scientific work needed to reduce gaps.
Risk characterization for chronic exposure to chemical hazards does not typically include estimates of the likelihood and severity of adverse health effects associated with different levels of exposure. A “notional zero risk” approach is generally taken and where possible the goal is to limit exposure to levels judged unlikely to have any adverse effects at all (see section 3.5.3 below).
3.5.2. Qualitative or quantitative?
Risk assessment outputs can range from qualitative to quantitative with various intermediate formats (see Figure 3.2). The characteristics of risk assessments presented above apply to all types. In qualitative risk assessments, outputs are expressed in descriptive terms such as high, medium or low. In quantitative risk assessments, the outputs are expressed numerically and may include a numerical description of uncertainty. In some cases, intermediate formats are referred to as semi-quantitative risk assessments. For instance, one semi-quantitative approach may be to assign scores at each step in the pathway and express outputs as risk rankings.3.5.2.1. Deterministic (point estimate) approaches
The term “deterministic” describes an approach in which numerical point values are used at each step in the risk assessment; for example, the mean or the 95th percentile value of measured data (such as food intake or residue levels) may be used to generate a single risk estimate. Deterministic approaches are the norm in chemical risk assessment, for instance to determine whether any risk may arise from consumption of a single food containing a chemical residue arising from a use governed by an MRL.
Figure 3.2. Continuum of risk assessment types
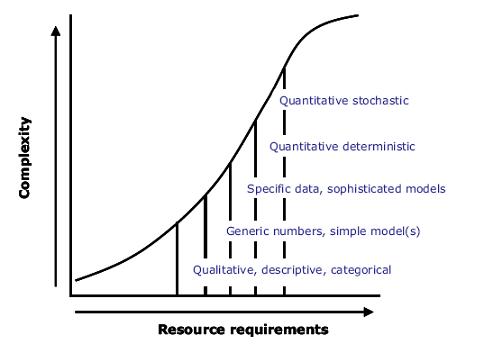
3.5.2.2. Stochastic (probabilistic) approaches
In stochastic approaches to risk assessment, scientific evidence is used to generate statements of probabilities of individual events, which are combined to determine the probability of an adverse health outcome. This requires mathematical modelling of the variability of the phenomena involved, and the final risk estimate is a probability distribution. Stochastic (probabilistic) models are then used to create and analyse different scenarios of risk. This approach is generally viewed as being most reflective of the real world, but stochastic models are often complex and difficult to generate.
Stochastic models are only now beginning to be used to complement the “safety evaluation” approaches traditionally used in managing chemical food-borne hazards, in particular for contaminants. On the other hand, probabilistic approaches are the norm in the newer discipline of microbial risk assessment and provide a mathematical description of the dynamics of hazard transmission from production to consumption. Exposure data are combined with dose-response information to generate probabilistic estimates of risk. Even one colony-forming unit of the pathogen in an edible portion of food is assumed to have some probability of causing infection; in this respect, such risk models resemble risk assessment methodology for chemical carcinogens.
3.5.3. Risk assessment for chemical hazards
Chemical hazards in foods include food additives, environmental contaminants such as mercury and dioxins, natural toxicants in food, such as glycoalkaloids in potatoes and aflatoxins in peanuts, acrylamide, and residues of pesticides and veterinary drugs. The scientific rationale for risk assessment of chemical hazards is somewhat different from that for biological hazards. Adverse health effects are usually predicted for long-term exposure to chemicals, whereas biological hazards are generally assessed in terms of a single exposure and an acute health risk19. For certain chemicals, such as some mycotoxins, marine toxins, pesticides and veterinary drugs, both acute and chronic health effects need to be considered.Considerable amounts of data of the types needed to establish standards have been provided by long-standing global data-gathering systems and other information sources specific to the class of chemical hazard under consideration, such as industry registration packages for pesticides and veterinary drugs or for food additives.
On the risk management side, many quantitative standards for chemical hazards in foods have been established by Codex and some national governments over several decades based on the mostly predictive risk assessment processes for chemicals. These generally employ a “worst case” standard-setting scenario based on a “notional zero risk” ALOP (for examples see Box 2.16 in Chapter 2 Risk management ).
3.5.3.1. Hazard identification
Hazard identification describes the adverse effects of the substance, the possibility of causing an adverse effect as an inherent property of the chemical, and the type (age group, gender, etc.) and extent of the population that may be at risk. Because sufficient human data from epidemiological studies are often not available, risk assessors frequently rely on results from toxicological studies in experimental animals and in vitro studies.
3.5.3.2. Hazard characterization
Hazard characterization describes and evaluates dose-response relationships for the most sensitive adverse effects reported in the available studies. This includes consideration of mechanistic aspects (e.g. whether the mechanism of action of the chemical observed in often high-dose experimental studies is also relevant to human exposure at lower levels).
In cases where the toxic effect results from a mechanism that has a threshold, hazard characterization usually results in the establishment of a safe level of intake, an acceptable
Note that many natural toxins such as mycotoxins and marine toxins need insight into biology as well as chemistry for their risk assessment.
daily intake (ADI), or tolerable daily intake (TDI) for contaminants. For some substances used as food additives the ADI may not need to be specified, i.e. no numerical ADI is considered necessary. This may be the case when a substance is assessed to be of very low toxicity, based on the biological and toxicological data, and the total dietary intake of the substance, arising from the levels permitted in foods to achieve the desired function does not represent a hazard.
Estimation of safe level of intake20
Estimation of the ADI or TDI (PTWI) includes the application of default “uncertainty factors” to a no-effect-level or low-effect level observed in experimental or epidemiological studies, to account for uncertainties inherent in extrapolating from an animal model to humans and to account for inter-individual variability (see Box 3.7). An ADI or TDI therefore represents a crude but conservative approximation of an actual chronic safe daily intake; both the estimate of risk and the inherent uncertainties remain unquantified. If sufficient data are available, the default uncertainty factors can be replaced by data-derived chemical-specific extrapolation factors. The term tolerable daily intake (TDI) or provisional tolerable weekly intake (PTWI), as opposed to an ADI, is used for contaminants and established by applying the same methods and principles.
The conservatism considered to be inherent in such a safety evaluation is generally thought to ensure sufficient protection of human health.
Methods have also been developed for calculating reference doses for acute exposures to toxic chemicals when such potential adverse health effects are plausible, even if rare. For example, an acute reference dose (ARfD) may be calculated for a pesticide to take into account the possibility of occasional intake of residues that far exceed the MRL.
Toxicological reference values used by different authorities for (genotoxic) carcinogenic chemicals vary. Some are based on a combination of epidemiological and animal data, some may be based on animal data alone, and different mathematical models may be used to extrapolate risk estimates to low doses. These differences can lead to significant variability in cancer risk estimates for the same chemical.
3.5.3.3. Exposure assessment
Exposure assessment describes the exposure pathway or pathways for a chemical hazard and estimates total intake. For some chemicals, intake may be associated with a single food, while for others the residue may be present in multiple foods, as well as in drinking water, and sometimes in household products, such that food accounts for only a portion of total exposure. For chemicals, exposure assessment often uses values at certain points on the continuum of exposure, such as the mean or the 97.5th percentile. Such point estimates are referred to as deterministic models. Some exposure models are emerging, such as for intake of pesticide residues, that take into account the distribution of food consumption by a population. These models, generally called probabilistic, provide more details on the distribution of exposed consumers, but are not inherently more accurate than deterministic models.
The outcome of the exposure assessment is compared to the ADI or TDI in order to determine whether estimated exposures to the chemical in foods are within safe limits.
These are toxicological reference values, or also called health-based guidance values.
3.5.3.4. Risk characterization
Risk characterization in chemical risk assessment primarily takes the form of defining a level of exposure presumed to pose a “notional zero risk.” That is, the ALOP is set below a dose associated with any significant likelihood of harm to health. Standards are then typically based on “worst case” exposure scenarios in order to keep risk below this ALOP.
Quantitative risk assessment methodologies have only rarely been applied for chemical hazards thought to pose no appreciable risk below certain very low levels of exposure (i.e. those with mechanisms of toxic action believed to exhibit a threshold), probably because the approach described above has generally been considered to provide an adequate margin of safety without a need to further characterize the risk. In contrast, quantitative risk assessment models have been applied by some governments as well as by international expert bodies (JECFA) for effects that are judged to have no threshold, i.e. for genotoxic carcinogens. These models employ biologically-appropriate mathematical extrapolations from observed animal cancer incidence data (usually derived from tests using high doses) to estimate the expected cancer incidence at the low levels typical of ordinary human exposure. If epidemiological cancer data are available, they also can be used in quantitative risk assessment models.
Annex 2 provides an example of chemical risk assessment methods applied to characterize the risk of a non-carcinogen, methylmercury, as a contaminant in fish.
3.5.3.5. Application of toxicological guidance values
For veterinary drug and pesticide residues, maximum residue levels (MRLs) are derived from controlled studies and are generally established so that the theoretical maximum daily intake of residues (calculated by any of several accepted methods) does not exceed the ADI.
For environmental contaminants and other chemicals that appear in food, regulatory standards often define “permissible levels” (or maximum levels (MLs) established by risk managers). In assessing the risks of these hazards it is recognized that as a practical matter it is often neither economically nor technically feasible to apply the same “notional zero risk” model to unavoidable contaminants as to other chemicals in the food supply. MLs are generally set so that the estimated intake does not exceed the TDI or PTWI. Risk managers may ask the risk assessors to compare the health protection impact of different proposed MLs. In such cases, the risk assessors focus on the exposure assessment to provide a more in-depth scientific basis for the risk management choices.
3.5.4. Risk assessment for biological hazards
Biological risk assessments typically use a quantitative model to describe the baseline food safety situation and estimate the level of consumer protection currently afforded. Then, some of the inputs into the model are changed, such as the level of the hazard in the raw food at the time of primary production, the conditions of processing, the temperature at which packaged material is held during retail and in the home. Changing inputs in a series of simulations enables the risk assessors to predict the impacts of the various control measures on the level of risk compared to that estimated in the baseline model.3.5.4.1. Hazard identification
A wide range of biological hazards can cause food-borne illness. Long-familiar hazards include microbes, viruses, parasites and toxins of biological origin, but new hazards are continually being identified, such as E. coli O157:H7, the prion agent of BSE, and multi-antibiotic resistant strains of Salmonella. In a given case, a risk profile may have identified specific strains or genotypes of pathogens that pose risks in a particular situation, and assessment may focus on these.
3.5.4.2. Hazard characterization
A wide range of hazard factors (e.g. infectivity, virulence, antibiotic resistance) and host factors (e.g. physiological susceptibility, immune status, previous exposure history, concurrent illness) affect hazard characterization and its associated variability. Epidemiological information is essential for full hazard characterization.
While dose-response data are essential for quantitative biological risk assessment, such data are often difficult to obtain for specific hazards. Relatively little human data is available to model dose-response curves for specific populations of interest, and assumptions often have to be made in this area, e.g. by using surrogate dose-response data from a different pathogen.
However, data from outbreak investigations can be a useful source in establishing the dose-response relationship.
Dose-response relationships can be developed for a range of human responses, e.g. infection, morbidity, hospitalization, and death rates associated with different doses.
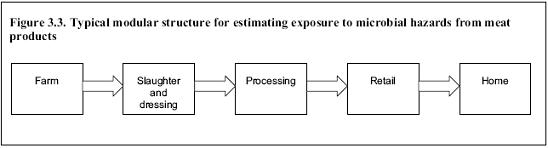
3.5.4.3. Exposure assessment
A food-chain exposure pathway model up to the point of consumption is developed for the hazard so that a human dose-response curve can be used to generate estimates of risk (Figure 3.3). Consideration of the whole food chain, while not always necessary, should be encouraged to the extent required to answer the risk managers’ questions. The level of human exposure depends on many factors including: the extent of initial contamination of the raw food, characteristics of the food and the food processes in terms of the hazard organism’s survival, multiplication or death, and storage and preparation conditions before eating. Some transmission pathways, for instance those for Campylobacter in poultry, may involve cross-contamination at retail or in the home.
3.5.4.4. Risk characterization
Risk estimates can be qualitative, e.g. high, medium or low rankings for a pathogen, or presented in quantitative terms, e.g. cumulative frequency distributions of risk per serving(s), annual risks for targeted populations, or relative risks for different foods or different pathogens.
Considerable challenges lie ahead in carrying out national quantitative microbial risk assessments for hazard-food combinations that pose significant risks to human health. Codex has stated in its guidelines for microbiological risk assessment that “a microbiological risk assessment should explicitly consider the dynamics of microbiological growth, survival, and death in foods and the complexity of the interaction (including sequelae) between human and agent following consumption as well as the potential for further spread”.21 However, biological characteristics of the pathogen/host relationship are often uncertain and modelling the exposure pathway from production to consumption often suffers from substantial data gaps.
Bearing these challenges in mind, risk characterization for microbial hazards may be somewhat inaccurate, but the greater strength of microbial risk assessment lies in its ability to model different food control measures and their impact on estimates of relative risks. Modelling “what-if” scenarios, such as changing the assumed prevalence of infection in the live animal population from which the food is derived, is also an essential part of economic analysis (see section 3.6).
Annex 3 provides an example of the use of microbial risk assessment in managing Listeria monocytogenes in ready-to-eat foods.
3.5.5. Biotechnology risk assessment
Risk analysis principles and food safety assessment guidelines have recently been elaborated by Codex for foods derived from “modern biotechnology”, i.e. those containing, derived from or produced using genetically modified organisms. Potential adverse health effects that require assessment include transfer of, or creation of new, toxins or allergens into foods with introduced genetic traits.Safety assessment is carried out to identify whether a hazard, nutritional or other safety concern is present, in which case information on its nature and severity should be collected and analysed. The safety assessment should include a comparison between the whole food derived from modern biotechnology (or component thereof) and its conventional counterpart, taking into account both intended and unintended effects.
If a new or altered hazard, nutritional or other safety concern is identified by the safety assessment, the risk associated with it should be characterized to determine its relevance to human health, using those testing and risk assessment methods appropriate to the nature of the identified concern. In this context, animal feeding studies may not be suitable as a test system to characterize risks arising from modern biotechnology, and a relatively broad range of other tests may need to be applied to fully assess the potential for risks to human health.
Pre-market safety assessments should be undertaken on a case-by-case basis using a structured and integrated approach.
3.5.6. Sensitivity analysis
Sensitivity analysis is a tool that can help risk managers select those controls that best achieve risk management goals. Sensitivity analysis, as a scientific process, shows the effects of changes in various inputs (data or assumptions) on the outcomes of a risk assessment. One of the most useful insights gained from a sensitivity analysis is estimating how much the uncertainty or variability associated with each input factor contributes to the overall uncertainty and variability in the risk estimate. Input distributions where uncertainty has the greatest impact on the outcome can be identified, and this process also can help set priorities for research to reduce uncertainty.3.5.7. Validation
Model validation is the process of evaluating a simulation model used in a risk assessment for its accuracy in representing a food safety system, e.g. by comparing model predictions of food-borne disease with human surveillance data, or by comparing model predictions on hazard levels at intermediate steps in the food production chain with actual monitoring data.While validation of the outputs of a risk assessment is desirable, this activity is not always practical. This is especially true for chemical risk assessments, where chronic adverse health effects in humans may be predicted from animal tests but can rarely be validated with human data.
3.5.8. Establishment of “targets” in the food chain as regulatory standards
The concept of setting food safety “targets” at various points in the food production chain as flexible implementation tools was described in Chapter 2. Developing and evaluating specific, quantitative microbiological metrics, such as performance objectives and performance criteria that can be incorporated in regulations, was described in Boxes 2.14 and 2.15.Risk assessors are involved in developing risk-based microbiological targets by simulating their impacts in risk models. In most cases, the goal of such simulations is to develop practical risk-based metrics than can be directly incorporated (and monitored) in HACCP plans, such as process criteria, product criteria and microbiological criteria. However, considerable methodological challenges remain in this area.
The concept of regulatory targets is equally applicable to chemical hazards. Currently, standards for chemical hazards in foods are often generic, such as requiring use of a pesticide or veterinary drug according to good agricultural practice (GAP) and good veterinary practice (GVP). MRLs developed from this process are not directly related to health outcomes. An appropriate performance target developed from a quantitative risk assessment could be the level of chemical hazard that is permissible at a specified step in the food chain, weighted relative to the ADI.
3.6. Integrating risk assessment and economic assessment
As both risk assessment and economic assessment suffer from uncertainty, there are real benefits in integrating the two disciplines to gain more realistic descriptions of the consequences of decisions that may be made by risk managers. The common element is being able to create a single matrix for decision-making. Typically, such matrices convert all outcomes, health impacts, economic costs and other costs, into units (such as dollars, “disability-adjusted life years”, DALYs, or “quality-adjusted life years”, QALYs) that permit ready comparison. While noting the increasing interest in using such tools, it is beyond the scope of this Guide to examine economic methodologies for estimating costs and benefits of different risk management options.Nevertheless, one good recent example of integrated risk assessment and economic assessment is the work of Havelaar and others in the Netherlands, who estimated cost-utility ratios for different interventions to reduce contamination of broiler chickens with Campylobacter. Figure 3.4, from their analysis, makes the cost per unit of health risk averted (DALY) very transparent to risk managers making decisions on control measures. It shows that decontamination in the scald tank, cooking (prepared meat) and good kitchen hygiene have by far the greatest cost-utility.
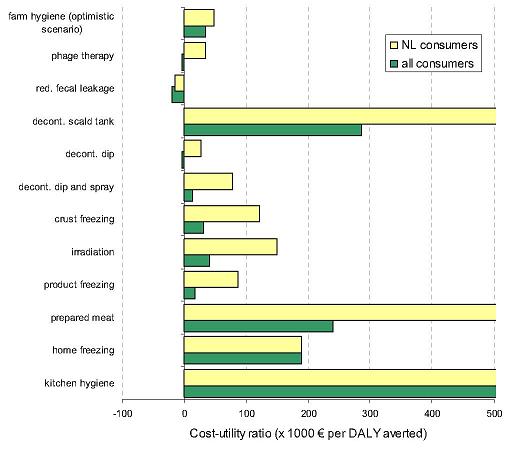
* Data are presented for effect on Dutch consumers (NL) and for effect on all consumers (including those who consume exports from the Netherlands), from Havelaar and others, 2005.
3.7. Suggestions for further reading
Batz, M.B., Morris, G., Painter, J., Singh, R., Tauxe, R.V. and others. 2005. Attributing illness to food. Emerging and Infectious Disease. 11: 993-999.European Commission. 2006. Towards an integrated approach in supporting microbial food safety decisions. RIVM Report 330050001/2006. RIVM, Bilthoven.
European Food Safety Authority. 2006. Transparency in risk assessment carried out by EFSA: Guidance document on procedural aspects. EFSA Journal (2006) 353, 1-16 (available at: http://www.efsa.europa.eu/en/science/sc_commitee/sc_documents/1494.html).
Evans, J.R. & Olson, D.L. 2002. Introduction to simulation and risk analysis. Prentice Hall, New Jersey.
FAO/WHO. 1997. Food consumption and exposure assessment of chemicals. Report of a FAO/WHO Consultation. Geneva, 10-14 February 1997.
FAO/WHO. 1999. Principles and Guidelines for the Conduct of Microbiological Risk Assessment. Codex Alimentarius Commission. CAC/GL 30-1999 (available at: http://www.codexalimentarius.net/web/standard_list.do?lang=en).
FAO/WHO. 2002. Risk assessments of Salmonella in eggs and broiler chickens. Microbiological Risk Assessment Series, No. 2 (available at: ftp://ftp.fao.org/docrep/fao/005/y4392e/y4392e00.pdf).
FAO/WHO. 2003. Guidelines for the Conduct of Food Safety Assessment of Foods Produced Using Recombinant-DNA microorganisms. Codex Alimentarius Commission. CAC/GL 46-2003 (available at: http://www.codexalimentarius.net/web/standard_list.do?lang=en).
FAO/WHO. 2003. Guideline for the Conduct of Food Safety Assessment of Foods Derived from Recombinant-DNA Plants. Codex Alimentarius Commission. CAC/GL 45-2003 (available at: http://www.codexalimentarius.net/web/standard_list.do?lang=en).
FAO/WHO. 2003. Hazard characterization for pathogens in food and water. Guidelines. Microbiological Risk Assessment Series, No. 3 (available at: ftp://ftp.fao.org/docrep/fao/006/y4666E/y4666E00.pdf).
FAO/WHO. 2004. Risk assessment of Listeria monocytogenes in ready-to-eat foods. Technical Report. Microbiological Risk Assessment Series, No. 5 (available at: http://www.fao.org/ag/agn/jemra/listeria_report_en.stm).
FAO/WHO. 2006. FAO/WHO Framework for the Provision of Scientific Advice on Food Safety and Nutrition (to Codex and member countries). (available at: http://www.fao.org/ag/agn/proscad/index_en.stm).
FAO/WHO. Project to update the principles and methods for the assessment of chemicals in food (available at: http://www.who.int/ipcs/food/principles/en/index.html).
FAO/WHO. 2006. A Model for Establishing Upper Levels of Intake for Nutrients and Related Substances. Report of a Joint FAO/WHO Technical Workshop on Nutrient Risk Assessment. Geneva, Switzerland. 2-6 May 2005 (available at: http://www.who.int/ipcs/highlights/full_report.pdf).
FAO/WHO. Risk assessments and publications of JECFA, JEMRA and JMPR are available on the FAO and WHO web sites:
JECFA: http://www.fao.org/ag/agn/jecfa/index_en.stmGruszczynski, L. 2006. The Role of Science in Risk Regulation under the SPS Agreement. Department of Law, European University Institute. EUI Law Working Paper No.2006/03 (available at: http://papers.ssrn.com/sol3/papers.cfm?abstract_id=891114).
http://www.who.int/ipcs/publications/jecfa/en/index.htmlJEMRA:http://www.fao.org/ag/agn/jemra/riskassessment_en.stm
http://www.who.int/foodsafety/micro/jemra/en/index.htmlJMPR: http://www.fao.org/ag/agp/agpp/pesticid/
http://www.who.int/ipcs/publications/jmpr/en/
Haas, C.N., Rose, J.B. & Gerba, C.P. 1999. Quantitative microbial risk assessment. John Wiley and Sons.
Havelaar, A.H., Nauta, M.J., Mangen, M.J., De Koeijer, A.G., Bogaardt, M.J., Evers, E.G., Jacobs-Reitsma, W.J., Van Pelt, W., Wagenaar, J., De Wit, G.A. & Van der Zee, H. 2005. Costs and benefits of controlling Campylobacter in the Netherlands – integrating risk analysis, epidemiology and economics. National Institute for Public Health and the Environment, Bilthoven. Report No. 250911009 (available at: http://www.rivm.nl/bibliotheek/rapporten/250911009.pdf).
National Research Council. 1994. Science and Judgment in Risk Assessment. Washington, DC: National Academy Press.
Paoli, G. 2005. Dimensions of validity in risk assessment. In Using microbiological risk assessment in food safety management. Report of workshop held in Prague, 12-14 October 2005. ILSI Europe, Brussels.
Vose, D. 2002. Risk analysis: a quantitative guide. Second edition. John Wiley and Sons, New York.
Weed, D.L. 2005. Weight of evidence: A review of concept and methods. Risk Analysis. Vol 25:1545-1557.
WHO. 1987. Principles for the Safety Assessment of Food Additives and Contaminants in Food. Environmental Health Criteria Document 70.
WHO. 1990. Principles for the Toxicological Assessment of Pesticide Residues in Food. Environmental Health Criteria Document 104.
WHO. 1993. Biomarkers and Risk Assessment: Concepts and Principles. Environmental Health Criteria Document 155.
WHO. 2006. Evaluation of Certain Food Contaminants. 64th Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 930 (available at: http://whqlibdoc.who.int/trs/WHO_TRS_930_eng.pdf).
Notes:
14
Epidemiology data are important for risk assessment. Epidemiology, as a tool, can also be used independently of risk assessment, for example in food source attribution (see section 2).
15
“Proportionality” means that control measures should be in proportion to the risk; e.g. if the risk assessment identifies negligible risks it is unreasonable to introduce an SPS measure that requires a stringent and costly regulatory regime.
16
The term “safety evaluation” is often used in regard to chemical hazards because the chief output is a definition of a presumptive “safe” exposure level, without detailed assessment of how risk varies with exposure to differing doses.
17
FAO/WHO. 2006. FAO/WHO Framework for the Provision of Scientific Advice on Food Safety and Nutrition (to Codex and member countries). Final draft for public comments (available at: ftp://ftp.fao.org/ag/agn/proscad/framework_en.pdf).
18
The Delphi method is a technique for eliciting and refining group judgements. The objective is generally the reliable and creative exploration of ideas or the production of suitable information for decision making (further information on this method is available at: http://www.iit.edu/~it/delphi.html).
19
Note that many natural toxins such as mycotoxins and marine toxins need insight into biology as well as chemistry for their risk assessment.
20
These are toxicological reference values, or also called health-based guidance values.
21
FAO/WHO. 1999. Principles and Guidelines for the Conduct of Microbiological Risk Assessment. Codex Alimentarius Commission. CAC/GL 30-1999 (available at:
http://www.codexalimentarius.net/web/standard_list.do?lang=en)
Source: FAO
 back to top
back to top



 companias
companias