This article is not yet available in the language you selected
Carbon dioxide
Here is a short summary of the principle of the potentiometric measurement of CO2 which this sensor and system is based on (Severinghaus). The CO2 sensor employs a gas permeable silicone membrane which is tightly stretched around a special engineered flat pH membrane. The CO2 from the sample or process diffuses through the membrane into or out the electrolyte where it equilibrates with HCO3- ions thus generating or consuming protons. The respective pH change of the electrolyte is sensed by a pH electrode and is logarithmically proportional to the partial pressure of carbon dioxide in the measuring solution.The dissolved CO2 gas reacts with water to form bicarbonate and H+ ions:
Relationship between partial pressure and solubility of CO2
Under equilibrium conditions we have the same partial pressure of CO2 on both sides of the gaspermeable membrane. It is important to understand that the CO2 sensor only measures the partial pressure of CO2 in the measuring solution. Other factors, such as pH value, salt concentration and temperature only have an indirect effect in so far as they influence the (pCO2) / (CO2) / (HCO3-) relationship in the measuring solution.If a liquid is saturated with a gas mixture, a dynamic equilibrium will be reached.
Analogy: A dynamic equilibrium is like a busy store that has customers continually arriving and leaving, with the number of customers inside the store remaining constant.
In the equilibrium state the partial pressure of a gas component will be equal in the liquid and in the gas phase.
According to Dalton's law, the total pressure p of a mixture of gases is the sum of the partial pressures of the components. These partial pressures are the pressures the gases would exert if they were alone in the container (at the same temperature).
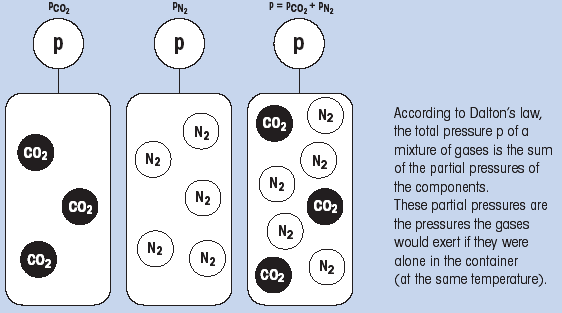
(Fig. 1: Dalton's law)
Example: Water at 25°C (77°F) is saturated with a gas mixture of 50% CO2 and 50% N2. Overall pressure is 1000 mbar. During saturation CO2 will react with water to form HCO3- ions before building-up a partial pressure (see figure 2).
Liquid phase contains water, dissolved CO2, dissolved N2, HCO3-, H+, OH- ions
Gas phase contains water vapor, CO2, N2.
Water vapor pressure is temperature dependent. At 25°C (77°F) P (H2O) = 32 mbar.
The partial pressures are therefore as follows:
PH2O = 32 mbar
PC02 = 484 mbar
PN2.........= 484 mbar
P (tot) = 1 000 mbar
Conclusion: The partial pressure of CO2 in liquid and gas phase is 484 mbar. The vapor pressure of water, as a function of temperature, must always be taken into consideration.
The partial pressure is the contribution that the gas makes to the total pressure of a sample.
The solubility of CO2 in water is proportional to its partial pressure (Henry's law), because an increase in pressure corresponds to an increase in the rate at which gas molecules strikes the surface of the solvent.
At 25°C (77°F) the Henry constant for CO2 is 1.402 g/l bar.
The CO2 solubility is much higher than the solubility of oxygen.
Example: same partial pressure for O2 and CO2 p = 212 mbar at 25°C (77°F)
Solubility O2 (dissolved oxygen) = 8.52 mg/l
Solubility CO2 (dissolved carbon dioxide) = 297.2 mg/l
The solubility of a gas in water is strongly temperature dependent.
The solubility increases with decreasing temperature.
CO2 saturation index SICO2 (%) is defined:
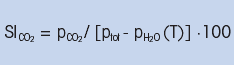
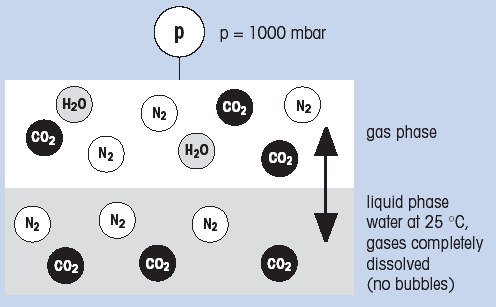
(Fig. 2: Equilibration between gas and liquid phase)
 back to top
back to top



 companias
companias